ODYSSEY FH I and FH II: 78 week results with alirocumab treatment in 735 patients with heterozygous familial hypercholesterolaemia
- PMID: 26330422
- PMCID: PMC4644253
- DOI: 10.1093/eurheartj/ehv370
ODYSSEY FH I and FH II: 78 week results with alirocumab treatment in 735 patients with heterozygous familial hypercholesterolaemia
Abstract
Aims: To assess long-term (78 weeks) alirocumab treatment in patients with heterozygous familial hypercholesterolaemia (HeFH) and inadequate LDL-C control on maximally tolerated lipid-lowering therapy (LLT).
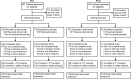
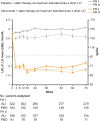
Methods and results: In two randomized, double-blind studies (ODYSSEY FH I, n = 486; FH II, n = 249), patients were randomized 2 : 1 to alirocumab 75 mg or placebo every 2 weeks (Q2W). Alirocumab dose was increased at Week 12 to 150 mg Q2W if Week 8 LDL-C was ≥1.8 mmol/L (70 mg/dL). Primary endpoint (both studies) was percentage change in calculated LDL-C from baseline to Week 24. Mean LDL-C levels decreased from 3.7 mmol/L (144.7 mg/dL) at baseline to 1.8 mmol/L (71.3 mg/dL; -57.9% vs. placebo) at Week 24 in patients randomized to alirocumab in FH I and from 3.5 mmol/L (134.6 mg/dL) to 1.8 mmol/L (67.7 mg/dL; -51.4% vs. placebo) in FH II (P < 0.0001). These reductions were maintained through Week 78. LDL-C <1.8 mmol/L (regardless of cardiovascular risk) was achieved at Week 24 by 59.8 and 68.2% of alirocumab-treated patients in FH I and FH II, respectively. Adverse events resulted in discontinuation in 3.4% of alirocumab-treated patients in FH I (vs. 6.1% placebo) and 3.6% (vs. 1.2%) in FH II. Rate of injection site reactions in alirocumab-treated patients was 12.4% in FH I and 11.4% in FH II (vs. 11.0 and 7.4% with placebo).
Conclusion: In patients with HeFH and inadequate LDL-C control at baseline despite maximally tolerated statin ± other LLT, alirocumab treatment resulted in significant LDL-C lowering and greater achievement of LDL-C target levels and was well tolerated.
Clinical trial registration: Cinicaltrials.gov (identifiers: NCT01623115; NCT01709500).
Keywords: Alirocumab; Cardiovascular risk; Heterozygous familial hypercholesterolaemia; LDL-C; PCSK9.
© The Author 2015. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures
Comment in
-
Simplified algorithm to facilitate communication of familial hypercholesterolaemia.Eur Heart J. 2015 Nov 14;36(43):3004-6. doi: 10.1093/eurheartj/ehv441. Epub 2015 Sep 1. Eur Heart J. 2015. PMID: 26330423 No abstract available.
References
-
- Nordestgaard BG, Chapman MJ, Humphries SE, Ginsberg HN, Masana L, Descamps OS, Wiklund O, Hegele RA, Raal FJ, Defesche JC, Wiegman A, Santos RD, Watts GF, Parhofer KG, Hovingh GK, Kovanen PT, Boileau C, Averna M, Boren J, Bruckert E, Catapano AL, Kuivenhoven JA, Pajukanta P, Ray K, Stalenhoef AF, Stroes E, Taskinen MR, Tybjaerg-Hansen A. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J 2013;34:3478–3490a. - PMC - PubMed
-
- Mortality in treated heterozygous familial hypercholesterolaemia: implications for clinical management. Scientific Steering Committee on behalf of the Simon Broome Register Group. Atherosclerosis 1999;142:105–112. - PubMed
-
- Pijlman AH, Huijgen R, Verhagen SN, Imholz BP, Liem AH, Kastelein JJ, Abbink EJ, Stalenhoef AF, Visseren FL. Evaluation of cholesterol lowering treatment of patients with familial hypercholesterolemia: a large cross-sectional study in The Netherlands. Atherosclerosis 2010;209:189–194. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

