Identifying growth hormone-regulated enhancers in the Igf1 locus
- PMID: 26330488
- PMCID: PMC4629005
- DOI: 10.1152/physiolgenomics.00062.2015
Identifying growth hormone-regulated enhancers in the Igf1 locus
Abstract
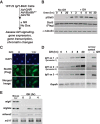
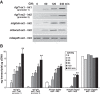
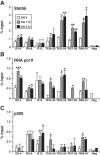
Growth hormone (GH) plays a central role in regulating somatic growth and in controlling multiple physiological processes in humans and other vertebrates. A key agent in many GH actions is the secreted peptide, IGF-I. As established previously, GH stimulates IGF-I gene expression via the Stat5b transcription factor, leading to production of IGF-I mRNAs and proteins. However, the precise mechanisms by which GH-activated Stat5b promotes IGF-I gene transcription have not been defined. Unlike other GH-regulated genes, there are no Stat5b sites near either of the two IGF-I gene promoters. Although dispersed GH-activated Stat5b binding elements have been mapped in rodent Igf1 gene chromatin, it is unknown how these distal sites might function as potential transcriptional enhancers. Here we have addressed mechanisms of regulation of IGF-I gene transcription by GH by generating cell lines in which the rat Igf1 chromosomal locus has been incorporated into the mouse genome. Using these cells we find that physiological levels of GH rapidly and potently activate Igf1 gene transcription while stimulating physical interactions in chromatin between inducible Stat5b-binding elements and the Igf1 promoters. We have thus developed a robust experimental platform for elucidating how dispersed transcriptional enhancers control Igf1 gene expression under different biological conditions.
Keywords: IGF-I; STAT; chromatin immunoprecipitation; epigenetics; gene transcription; growth hormone.
Copyright © 2015 the American Physiological Society.
Figures



Similar articles
-
Defining human insulin-like growth factor I gene regulation.Am J Physiol Endocrinol Metab. 2016 Aug 1;311(2):E519-29. doi: 10.1152/ajpendo.00212.2016. Epub 2016 Jul 12. Am J Physiol Endocrinol Metab. 2016. PMID: 27406741 Free PMC article.
-
Dispersed Chromosomal Stat5b-binding elements mediate growth hormone-activated insulin-like growth factor-I gene transcription.J Biol Chem. 2010 Jun 4;285(23):17636-47. doi: 10.1074/jbc.M110.117697. Epub 2010 Apr 7. J Biol Chem. 2010. PMID: 20378540 Free PMC article.
-
Biochemical characterization of diverse Stat5b-binding enhancers that mediate growth hormone-activated insulin-like growth factor-I gene transcription.PLoS One. 2012;7(11):e50278. doi: 10.1371/journal.pone.0050278. Epub 2012 Nov 20. PLoS One. 2012. PMID: 23185594 Free PMC article.
-
Mapping the growth hormone--Stat5b--IGF-I transcriptional circuit.Trends Endocrinol Metab. 2012 Apr;23(4):186-93. doi: 10.1016/j.tem.2012.01.001. Epub 2012 Feb 21. Trends Endocrinol Metab. 2012. PMID: 22361342 Free PMC article. Review.
-
Regulation of gene expression by growth hormone.Mol Cell Endocrinol. 2020 May 1;507:110788. doi: 10.1016/j.mce.2020.110788. Epub 2020 Mar 6. Mol Cell Endocrinol. 2020. PMID: 32151566 Free PMC article. Review.
Cited by
-
Defining human insulin-like growth factor I gene regulation.Am J Physiol Endocrinol Metab. 2016 Aug 1;311(2):E519-29. doi: 10.1152/ajpendo.00212.2016. Epub 2016 Jul 12. Am J Physiol Endocrinol Metab. 2016. PMID: 27406741 Free PMC article.
-
Insulinlike Growth Factor 1 Gene Variation in Vertebrates.Endocrinology. 2018 Jun 1;159(6):2288-2305. doi: 10.1210/en.2018-00259. Endocrinology. 2018. PMID: 29697760 Free PMC article.
-
Roles of differential expression of miR-543-5p in GH regulation in rat anterior pituitary cells and GH3 cells.PLoS One. 2019 Sep 11;14(9):e0222340. doi: 10.1371/journal.pone.0222340. eCollection 2019. PLoS One. 2019. PMID: 31509580 Free PMC article.
-
The New Genomics: What Molecular Databases Can Tell Us About Human Population Variation and Endocrine Disease.Endocrinology. 2017 Jul 1;158(7):2035-2042. doi: 10.1210/en.2017-00338. Endocrinology. 2017. PMID: 28498917 Free PMC article. Review.
-
Human growth hormone and human prolactin function as autocrine/paracrine promoters of progression of hepatocellular carcinoma.Oncotarget. 2016 May 17;7(20):29465-79. doi: 10.18632/oncotarget.8781. Oncotarget. 2016. PMID: 27102295 Free PMC article.
References
-
- Adamo ML, Ben-Hur H, Roberts CT Jr, LeRoith D. Regulation of start site usage in the leader exons of the rat insulin-like growth factor-I gene by development, fasting, and diabetes. Mol Endocrinol 5: 1677–1686, 1991. - PubMed
-
- Benbassat C, Shoba LN, Newman M, Adamo ML, Frank SJ, Lowe WLJ. Growth hormone-mediated regulation of insulin-like growth factor I promoter activity in C6 glioma cells. Endocrinology 140: 3073–3081, 1999. - PubMed
-
- Bichell DP, Kikuchi K, Rotwein P. Growth hormone rapidly activates insulin-like growth factor I gene transcription in vivo. Mol Endocrinol 6: 1899–1908, 1992. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

