Motor Learning and the Cerebellum
- PMID: 26330521
- PMCID: PMC4563713
- DOI: 10.1101/cshperspect.a021683
Motor Learning and the Cerebellum
Abstract
Although our ability to store semantic declarative information can nowadays be readily surpassed by that of simple personal computers, our ability to learn and express procedural memories still outperforms that of supercomputers controlling the most advanced robots. To a large extent, our procedural memories are formed in the cerebellum, which embodies more than two-thirds of all neurons in our brain. In this review, we will focus on the emerging view that different modules of the cerebellum use different encoding schemes to form and express their respective memories. More specifically, zebrin-positive zones in the cerebellum, such as those controlling adaptation of the vestibulo-ocular reflex, appear to predominantly form their memories by potentiation mechanisms and express their memories via rate coding, whereas zebrin-negative zones, such as those controlling eyeblink conditioning, appear to predominantly form their memories by suppression mechanisms and express their memories in part by temporal coding using rebound bursting. Together, the different types of modules offer a rich repertoire to acquire and control sensorimotor processes with specific challenges in the spatiotemporal domain.
Copyright © 2015 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures

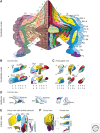


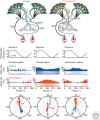

References
-
- Albus JS. 1971. A theory of cerebellar function. Math Biosci 10: 25–61.
-
- Angaut P, Compoint C, Buisseret-Delmas C, Batini C. 1996. Synaptic connections of Purkinje cell axons with nucleocortical neurones in the cerebellar medial nucleus of the rat. Neurosci Res 26: 345–348. - PubMed
-
- Apps R, Hawkes R. 2009. Cerebellar cortical organization: A one-map hypothesis. Nat Rev Neurosci 10: 670–681. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
