Identification and characterization of modified antisense oligonucleotides targeting DMPK in mice and nonhuman primates for the treatment of myotonic dystrophy type 1
- PMID: 26330536
- PMCID: PMC4613955
- DOI: 10.1124/jpet.115.226969
Identification and characterization of modified antisense oligonucleotides targeting DMPK in mice and nonhuman primates for the treatment of myotonic dystrophy type 1
Abstract
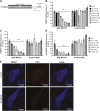
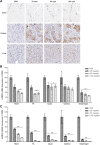
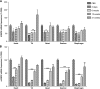
Myotonic dystrophy type 1 (DM1) is the most common form of muscular dystrophy in adults. DM1 is caused by an expanded CTG repeat in the 3'-untranslated region of DMPK, the gene encoding dystrophia myotonica protein kinase (DMPK). Antisense oligonucleotides (ASOs) containing 2',4'-constrained ethyl-modified (cEt) residues exhibit a significantly increased RNA binding affinity and in vivo potency relative to those modified with other 2'-chemistries, which we speculated could translate to enhanced activity in extrahepatic tissues, such as muscle. Here, we describe the design and characterization of a cEt gapmer DMPK ASO (ISIS 486178), with potent activity in vitro and in vivo against mouse, monkey, and human DMPK. Systemic delivery of unformulated ISIS 486718 to wild-type mice decreased DMPK mRNA levels by up to 90% in liver and skeletal muscle. Similarly, treatment of either human DMPK transgenic mice or cynomolgus monkeys with ISIS 486178 led to up to 70% inhibition of DMPK in multiple skeletal muscles and ∼50% in cardiac muscle in both species. Importantly, inhibition of DMPK was well tolerated and was not associated with any skeletal muscle or cardiac toxicity. Also interesting was the demonstration that the inhibition of DMPK mRNA levels in muscle was maintained for up to 16 and 13 weeks post-treatment in mice and monkeys, respectively. These results demonstrate that cEt-modified ASOs show potent activity in skeletal muscle, and that this attractive therapeutic approach warrants further clinical investigation to inhibit the gain-of-function toxic RNA underlying the pathogenesis of DM1.
Copyright © 2015 by The American Society for Pharmacology and Experimental Therapeutics.
Figures


References
-
- Agrawal S, Zhao Q, Jiang Z, Oliver C, Giles H, Heath J, Serota D. (1997) Toxicologic effects of an oligodeoxynucleotide phosphorothioate and its analogs following intravenous administration in rats. Antisense Nucleic Acid Drug Dev 7:575–584. - PubMed
-
- Bennett CF, Swayze EE. (2010) RNA targeting therapeutics: molecular mechanisms of antisense oligonucleotides as a therapeutic platform. Annu Rev Pharmacol Toxicol 50:259–293. - PubMed
-
- Berul CI, Maguire CT, Gehrmann J, Reddy S. (2000) Progressive atrioventricular conduction block in a mouse myotonic dystrophy model. J Interv Card Electrophysiol 4:351–358. - PubMed
-
- Blake DJ, Weir A, Newey SE, Davies KE. (2002) Function and genetics of dystrophin and dystrophin-related proteins in muscle. Physiol Rev 82:291–329. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

