The Social Amoeba Dictyostelium discoideum Is Highly Resistant to Polyglutamine Aggregation
- PMID: 26330554
- PMCID: PMC4646202
- DOI: 10.1074/jbc.M115.676247
The Social Amoeba Dictyostelium discoideum Is Highly Resistant to Polyglutamine Aggregation
Abstract
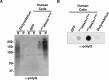
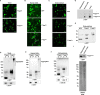
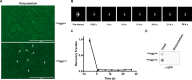
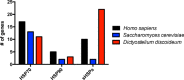
The expression, misfolding, and aggregation of long repetitive amino acid tracts are a major contributing factor in a number of neurodegenerative diseases, including C9ORF72 amyotrophic lateral sclerosis/frontotemporal dementia, fragile X tremor ataxia syndrome, myotonic dystrophy type 1, spinocerebellar ataxia type 8, and the nine polyglutamine diseases. Protein aggregation is a hallmark of each of these diseases. In model organisms, including yeast, worms, flies, mice, rats, and human cells, expression of proteins with the long repetitive amino acid tracts associated with these diseases recapitulates the protein aggregation that occurs in human disease. Here we show that the model organism Dictyostelium discoideum has evolved to normally encode long polyglutamine tracts and express these proteins in a soluble form. We also show that Dictyostelium has the capacity to suppress aggregation of a polyglutamine-expanded Huntingtin construct that aggregates in other model organisms tested. Together, these data identify Dictyostelium as a novel model organism with the capacity to suppress aggregation of proteins with long polyglutamine tracts.
Keywords: Dictyostelium; neurodegenerative disease; polyglutamine; protein aggregation; protein folding.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
-
- Duennwald M. L. (2013) Yeast as a platform to explore polyglutamine toxicity and aggregation. Methods Mol. Biol. 1017, 153–161 - PubMed
-
- Klement I. A., Skinner P. J., Kaytor M. D., Yi H., Hersch S. M., Clark H. B., Zoghbi H. Y., Orr H. T. (1998) Ataxin-1 nuclear localization and aggregation: role in polyglutamine-induced disease in SCA1 transgenic mice. Cell 95, 41–53 - PubMed
-
- Paulson H. L., Perez M. K., Trottier Y., Trojanowski J. Q., Subramony S. H., Das S. S., Vig P., Mandel J. L., Fischbeck K. H., Pittman R. N. (1997) Intranuclear inclusions of expanded polyglutamine protein in spinocerebellar ataxia type 3. Neuron 19, 333–344 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

