Isolation and Characterization of a New Thermoalkalophilic Lipase from Soil Bacteria
- PMID: 26330879
- PMCID: PMC4518119
Isolation and Characterization of a New Thermoalkalophilic Lipase from Soil Bacteria
Abstract
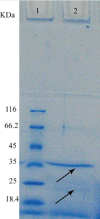
Lipases are diversified enzymes in their properties and substrate specificity, which make them attractive tools for various industrial applications. In this study, an alkalinethermostable lipase producing bacteria were isolated from soil of different regions of Isfahan province (Iran) and its lipase was purified by ammonium sulfate precipitation and ion exchange chromatography. To select a thermoalkalophil lipase producing bacterium, Rhodamine B and Horikoshi media were used and the strain that can grow at 45° Cwas selected. The isolated strain was identified using microbial and biochemical tests. One strain showed an orange colored zone on plate and grew on Horikoshi plate. Microbial and biochemical tests showed that the isolated strain was Bacillus subtilis, a Gram positive rod. In PCR, an expected band was obtained with about 371 bp. The activity of the purified lipase was 10.2 folds that of the standard enzyme using ammonium sulfate precipitation and ion exchange chromatography. The molecular weight of lipase determined by SDS-PAGE electrophoresis, was 21 and 35 KDa. Existence of two bands in SDS-PAGE electrophoresis and low amount of obtained purified enzyme highlights the necessity of optimization of purification and concentrating process.
Keywords: Bacillus subtilis; Lipase; Soil; Thermoalkalophile.
Figures


References
-
- Martinez A, Soberon-Chavez G. Characterization of the lipA gene encoding the major lipase from Pseudomonas aeruginosa strain IGB83. Appl. Microbiol. Biotechnol. 2001;56:731–735. - PubMed
-
- Saranya P, Kumari HS, Rao BP, Sekaran G. Lipase production from a novel thermo-tolerant and extreme acidophile Bacillus pumilus using palm oil as the substrate and treatment of palm oil-containing wastewater. Envir. Sci. Poll. Res. 2014;21:3907–3919. - PubMed
-
- Eggert T, Pencreac'h G, Douchet I, Verger R, Jaeger KE. A novel extracellular esterase from Bacillus subtilis and its conversion to a monoacylglycerol hydrolase. Eur. J. Biochem. 2000;267:6459–6469. - PubMed
-
- Treichel H, de Oliveira D, Mazutti MA, Di Luccio M, Oliveira JV. A review on microbial lipases production. Food Bioprocess Tech. 2010;3:182–196.
-
- Balan A, Magalinggam N, Ibrahim D, Rahim RA. Thermostable lipase production by Geobacillus thermodenitrificans in a 5-L stirred-tank bioreactor. Int. J. Microbiol. 2010;8:2–9.
LinkOut - more resources
Full Text Sources
