Engineered biomaterials for development of nucleic acid vaccines
- PMID: 26331076
- PMCID: PMC4552455
- DOI: 10.1186/s40824-014-0025-8
Engineered biomaterials for development of nucleic acid vaccines
Abstract
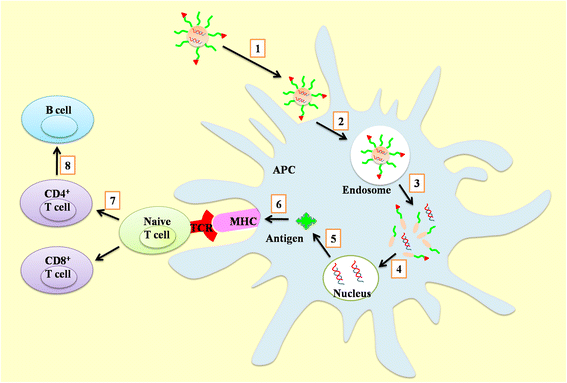
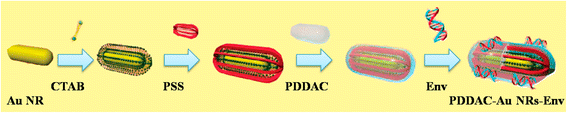
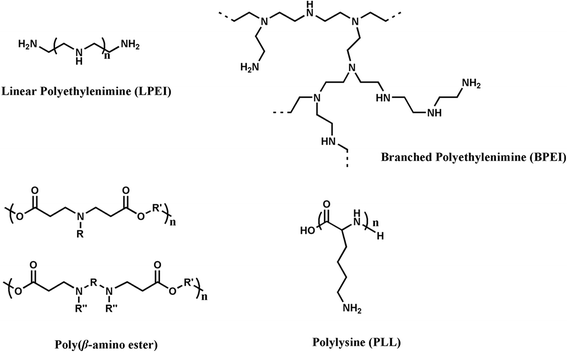
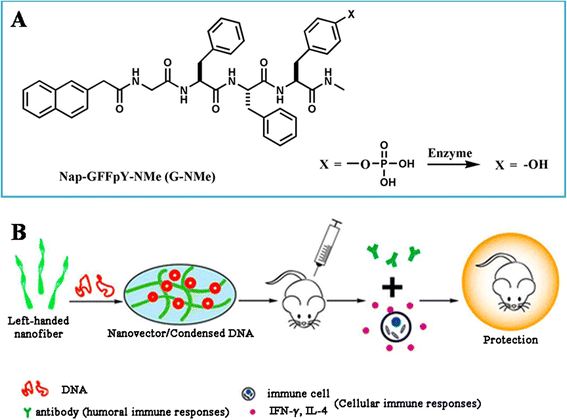
Nucleic acid vaccines have attracted many attentions since they have presented some superiority over traditional vaccines. However, they could only induce moderate immunogenicity. The route and formulation of nucleic acid vaccines have strong effects on the immune response and efficiency. Numerous biomaterials are used as a tool to enhance the immunogenicity of antigens. They deliver the antigens into the cells through particle- and non-particle-mediated pathway. However, challenges remain due to lack of comprehensive understanding of the actions of these biomaterials as a carrier/adjuvant. Herein, this review focuses on the evolution of biomaterials used for nucleic acid vaccines, discusses the advantages and disadvantages for gene delivery and immunostimulation of variety of structures of the biomaterials, in order to provide new thought on rational design of carrier/adjuvant and better understanding of mechanism of action in both immunostimulatory and delivery methods.
Keywords: Adjuvant; Biomaterials; Gene delivery; Immunogenicity; Nucleic acid vaccine.
Figures
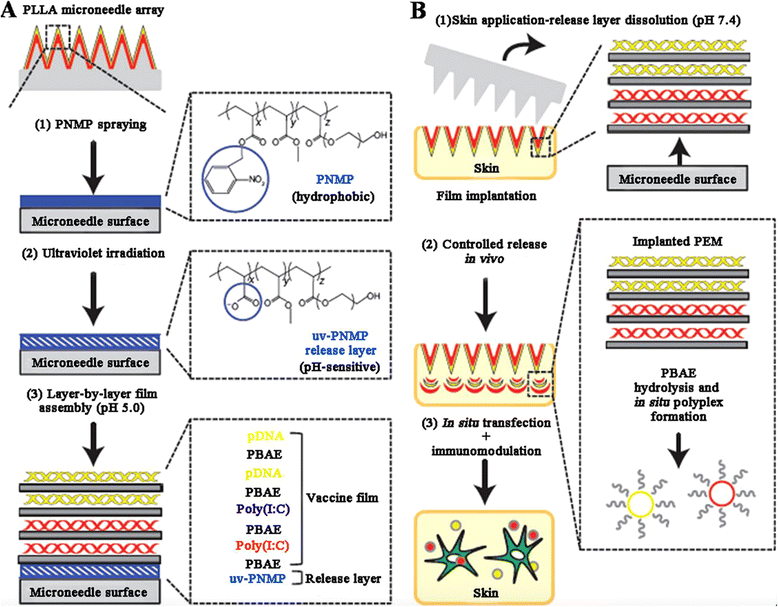
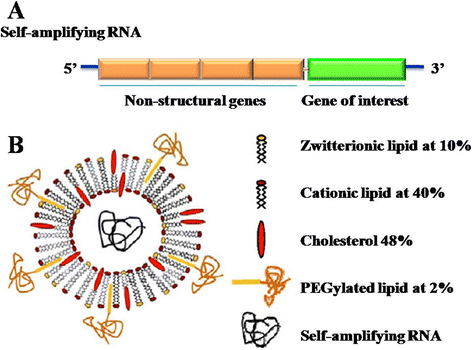
References
-
- Jenner E. An inquiry into the causes and effects of the variolae vaccinae, a disease discovered in some of the western counties of England, particularly Gloucestershire, and known by the name of the cow pox. 1798, New York: General Books.
-
- Kubba AK, Taylor P, Graneek B, Strobel S. Non-responders to hepatitis B vaccination: a review. Commun Dis Public Health. 2003;6:106–12. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

