Two-Photon Lifetime Imaging of Voltage Indicating Proteins as a Probe of Absolute Membrane Voltage
- PMID: 26331249
- PMCID: PMC4564826
- DOI: 10.1016/j.bpj.2015.07.038
Two-Photon Lifetime Imaging of Voltage Indicating Proteins as a Probe of Absolute Membrane Voltage
Abstract
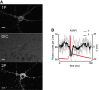
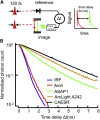
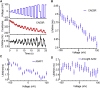
Genetically encoded voltage indicators (GEVIs) can report cellular electrophysiology with high resolution in space and time. Two-photon (2P) fluorescence has been explored as a means to image voltage in tissue. Here, we used the 2P electronic excited-state lifetime to probe absolute membrane voltage in a manner that is insensitive to the protein expression level, illumination intensity, or photon detection efficiency. First, we tested several GEVIs for 2P brightness, response speed, and voltage sensitivity. ASAP1 and a previously described citrine-Arch electrochromic Förster resonance energy transfer sensor (dubbed CAESR) showed the best characteristics. We then characterized the voltage-dependent lifetime of ASAP1, CAESR, and ArcLight under voltage-clamp conditions. ASAP1 and CAESR showed voltage-dependent lifetimes, whereas ArcLight did not. These results establish 2P fluorescence lifetime imaging as a viable means of measuring absolute membrane voltage. We discuss the prospects and improvements necessary for applications in tissue.
Copyright © 2015 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Fisher J.A., Barchi J.R., Salzberg B.M. Two-photon excitation of potentiometric probes enables optical recording of action potentials from mammalian nerve terminals in situ. J. Neurophysiol. 2008;99:1545–1553. - PubMed
-
- Cohen L.B., Salzberg B.M., Wang C.H. Changes in axon fluorescence during activity: molecular probes of membrane potential. J. Membr. Biol. 1974;19:1–36. - PubMed
-
- Salzberg B.M., Grinvald A., Ross W.N. Optical recording of neuronal activity in an invertebrate central nervous system: simultaneous monitoring of several neurons. J. Neurophysiol. 1977;40:1281–1291. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

