PE and PS Lipids Synergistically Enhance Membrane Poration by a Peptide with Anticancer Properties
- PMID: 26331251
- PMCID: PMC4564682
- DOI: 10.1016/j.bpj.2015.07.033
PE and PS Lipids Synergistically Enhance Membrane Poration by a Peptide with Anticancer Properties
Abstract
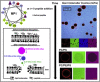
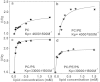
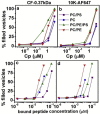
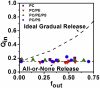
Polybia-MP1 (MP1) is a bioactive host-defense peptide with known anticancer properties. Its activity is attributed to excess serine (phosphatidylserine (PS)) on the outer leaflet of cancer cells. Recently, higher quantities of phosphatidylethanolamine (PE) were also found at these cells' surface. We investigate the interaction of MP1 with model membranes in the presence and absence of POPS (PS) and DOPE (PE) to understand the role of lipid composition in MP1's anticancer characteristics. Indeed we find that PS lipids significantly enhance the bound concentration of peptide on the membrane by a factor of 7-8. However, through a combination of membrane permeability assays and imaging techniques we find that PE significantly increases the susceptibility of the membrane to disruption by these peptides and causes an order-of-magnitude increase in membrane permeability by facilitating the formation of larger transmembrane pores. Significantly, atomic-force microscopy imaging reveals differences in the pore formation mechanism with and without the presence of PE. Therefore, PS and PE lipids synergistically combine to enhance membrane poration by MP1, implying that the combined enrichment of both these lipids in the outer leaflet of cancer cells is highly significant for MP1's anticancer action. These mechanistic insights could aid development of novel chemotherapeutics that target pathological changes in the lipid composition of cancerous cells.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Phosphatidylserine lipids and membrane order precisely regulate the activity of Polybia-MP1 peptide.Biochim Biophys Acta Biomembr. 2017 Jun;1859(6):1067-1074. doi: 10.1016/j.bbamem.2017.03.002. Epub 2017 Mar 6. Biochim Biophys Acta Biomembr. 2017. PMID: 28274844
-
The insertion of Polybia-MP1 peptide into phospholipid monolayers is regulated by its anionic nature and phase state.Chem Phys Lipids. 2017 Oct;207(Pt A):38-48. doi: 10.1016/j.chemphyslip.2017.08.001. Epub 2017 Aug 10. Chem Phys Lipids. 2017. PMID: 28802697
-
Influence of the bilayer composition on the binding and membrane disrupting effect of Polybia-MP1, an antimicrobial mastoparan peptide with leukemic T-lymphocyte cell selectivity.Biochemistry. 2012 Jun 19;51(24):4898-908. doi: 10.1021/bi201608d. Epub 2012 Jun 6. Biochemistry. 2012. PMID: 22630563
-
Platelet lipidomic.J Biol Regul Homeost Agents. 2012 Apr-Jun;26(2 Suppl 1):23S-33S. J Biol Regul Homeost Agents. 2012. PMID: 23648196 Review.
-
Role of lipids in the interaction of antimicrobial peptides with membranes.Prog Lipid Res. 2012 Apr;51(2):149-77. doi: 10.1016/j.plipres.2011.12.005. Epub 2012 Jan 8. Prog Lipid Res. 2012. PMID: 22245454 Review.
Cited by
-
Bioactive peptides: an alternative therapeutic approach for cancer management.Front Immunol. 2024 Jan 24;15:1310443. doi: 10.3389/fimmu.2024.1310443. eCollection 2024. Front Immunol. 2024. PMID: 38327525 Free PMC article. Review.
-
Advances in Lipid and Metal Nanoparticles for Antimicrobial Peptide Delivery.Pharmaceutics. 2019 Nov 8;11(11):588. doi: 10.3390/pharmaceutics11110588. Pharmaceutics. 2019. PMID: 31717337 Free PMC article. Review.
-
Itch in Hymenoptera Sting Reactions.Front Allergy. 2021 Aug 20;2:727776. doi: 10.3389/falgy.2021.727776. eCollection 2021. Front Allergy. 2021. PMID: 35387042 Free PMC article.
-
Posing for a picture: vesicle immobilization in agarose gel.Sci Rep. 2016 May 3;6:25254. doi: 10.1038/srep25254. Sci Rep. 2016. PMID: 27140695 Free PMC article.
-
Antimicrobial Peptide Analogs From Scorpions: Modifications and Structure-Activity.Front Mol Biosci. 2022 May 26;9:887763. doi: 10.3389/fmolb.2022.887763. eCollection 2022. Front Mol Biosci. 2022. PMID: 35712354 Free PMC article. Review.
References
-
- de Souza B.M., da Silva A.V., Palma M.S. Characterization of two novel polyfunctional mastoparan peptides from the venom of the social wasp Polybia paulista. Peptides. 2009;30:1387–1395. - PubMed
-
- Wang K.R., Zhang B.Z., Wang R. Antitumor effects, cell selectivity and structure-activity relationship of a novel antimicrobial peptide polybia-MPI. Peptides. 2008;29:963–968. - PubMed
-
- Wang K.R., Yan J.X., Wang R. Novel mode of action of polybia-MPI, a novel antimicrobial peptide, in multi-drug resistant leukemic cells. Cancer Lett. 2009;278:65–72. - PubMed
-
- dos Santos Cabrera M.P., Arcisio-Miranda M., Palma M.S. Influence of the bilayer composition on the binding and membrane disrupting effect of Polybia-MP1, an antimicrobial mastoparan peptide with leukemic T-lymphocyte cell selectivity. Biochemistry. 2012;51:4898–4908. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

