The Ras G Domain Lacks the Intrinsic Propensity to Form Dimers
- PMID: 26331257
- PMCID: PMC4564675
- DOI: 10.1016/j.bpj.2015.07.020
The Ras G Domain Lacks the Intrinsic Propensity to Form Dimers
Abstract
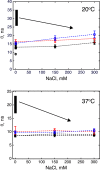
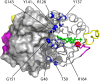
Ras GTPase is a molecular switch controlling a number of cellular pathways including growth, proliferation, differentiation, and apoptosis. Recent reports indicated that Ras undergoes dimerization at the membrane surface through protein-protein interactions. If firmly established this property of Ras would require profound reassessment of a large amount of published data and modification of the Ras signaling paradigm. One proposed mechanism of dimerization involves formation of salt bridges between the two GTPase domains (G domains) leading to formation of a compact dimer as observed in Ras crystal structures. In this work, we interrogated the intrinsic ability of Ras to self-associate in solution by creating conditions of high local concentration through irreversibly tethering the two G domains together at their unstructured C-terminal tails. We evaluated possible self-association in this inverted tandem conjugate via analysis of the time-domain fluorescence anisotropy and NMR chemical shift perturbations. We did not observe the increased rotational correlation time expected for the G domain dimer. Variation of the ionic strength (to modulate stability of the salt bridges) did not affect the rotational correlation time in the tandem further supporting independent rotational diffusion of two G domains. In a parallel line of experiments to detect and map weak self-association of the G domains, we analyzed NMR chemical shifts perturbations at a number of sites near the crystallographic dimer interface. The nearly complete lack of chemical shift perturbations in the tandem construct supported a simple model with the independent G domains repelled from each other by their overall negative charge. These results lead us to the conclusion that self-association of the G domains cannot be responsible for homodimerization of Ras reported in the literature.
Copyright © 2015 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Takai Y., Sasaki T., Matozaki T. Small GTP-binding proteins. Physiol. Rev. 2001;81:153–208. - PubMed
-
- Wittinghofer A., Vetter I.R. Structure-function relationships of the G domain, a canonical switch motif. In: Kornberg R.D., Raetz C.R.H., Rothman J.E., Thorner J.W., editors. Annual Review of Biochemistry. Annual Reviews; Palo Alto, CA: 2011. pp. 943–971. - PubMed
-
- Lowy D.R., Willumsen B.M. Function and regulation of ras. Annu. Rev. Biochem. 1993;62:851–891. - PubMed
-
- Vojtek A.B., Der C.J. Increasing complexity of the Ras signaling pathway. J. Biol. Chem. 1998;273:19925–19928. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

