Combination therapy with nifedipine GITS 60 mg: subanalysis of a prospective, 12-week observational study (AdADOSE)
- PMID: 26331311
- PMCID: PMC4720040
- DOI: 10.3109/10641963.2015.1060986
Combination therapy with nifedipine GITS 60 mg: subanalysis of a prospective, 12-week observational study (AdADOSE)
Abstract
Background: AdADOSE was a 12-week, international, observational study conducted in the Middle East and Russia where patients received nifedipine gastrointestinal therapeutic system (GITS) at a daily dose of 30, 60, or 90 mg as part of an antihypertensive combination therapy. This subgroup analysis of the AdADOSE study assesses the efficacy and tolerability of nifedipine GITS combination therapy when used specifically at the 60-mg strength.
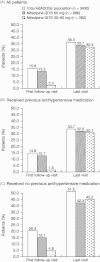
Methods: Patients with hypertension who received a daily nifedipine GITS dose of 60 mg, either at constant dose (n = 686) or up-titrated from 30 mg (n = 392), were analyzed. Target blood pressure (BP) was <140/90 mmHg (or <130/80 mmHg for those at high/very high cardiovascular risk).
Results: Following nifedipine GITS combination therapy, target BP was achieved by 33.7% patients in the 60 mg group (previously untreated, 42.5%; previously treated, 32.0%) and 32.4% patients in the 30-60 mg group (previously untreated, 45.2%; previously treated, 30.7%). Mean systolic BP/diastolic BP changes were -40.3/-20.7 mmHg and -35.6/-18.5 mmHg, respectively, and were similar regardless of previous antihypertensive treatment or the number of concomitant diseases. Incidences of drug-related adverse events (AEs) were low (3.2%, 60 mg; 2.0%, 30-60 mg group), few patients discontinued because of AEs (0.6% and 1.0%, respectively), and there were no serious AEs.
Conclusion: Combination therapy with nifedipine GITS 60 mg in a real-life observational setting was effective and well tolerated in hypertensive patients, with low rates of treatment-related AEs.
Keywords: AdADOSE; cardiovascular; hypertension; nifedipine GITS; observational study.
Figures

References
-
- Mancia G, DeBacker G, Dominiczak A, et al. 2007 Guidelines for the Management of Arterial Hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2007;25:1105–87. - PubMed
-
- Brennan F, Flanagan M, Blake S, Cannon P. Nifedipine in the treatment of hypertension. Eur J Clin Pharmacol. 1983;25:713–15. - PubMed
-
- Gradman AH, Basile JN, Carter BL, et al. Combination therapy in hypertension. J Am Soc Hypertens. 2010;4:90–8. - PubMed
-
- Poole-Wilson PA, Lubsen J, Kirwan BA, et al. Effect of long-acting nifedipine on mortality and cardiovascular morbidity in patients with stable angina requiring treatment (ACTION trial): randomised controlled trial. Lancet. 2004;364:849–57. - PubMed
-
- Hasebe N, Kikuchi K. Controlled-release nifedipine and candesartan low-dose combination therapy in patients with essential hypertension: the NICE Combi (Nifedipine and Candesartan Combination) Study. J Hypertens. 2005;23:445–53. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
