Distinct EMT programs control normal mammary stem cells and tumour-initiating cells
- PMID: 26331542
- PMCID: PMC4764075
- DOI: 10.1038/nature14897
Distinct EMT programs control normal mammary stem cells and tumour-initiating cells
Abstract
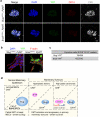
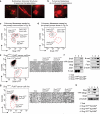
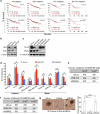
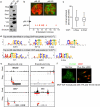
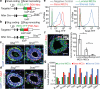
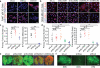
Tumour-initiating cells (TICs) are responsible for metastatic dissemination and clinical relapse in a variety of cancers. Analogies between TICs and normal tissue stem cells have led to the proposal that activation of the normal stem-cell program within a tissue serves as the major mechanism for generating TICs. Supporting this notion, we and others previously established that the Slug epithelial-to-mesenchymal transition-inducing transcription factor (EMT-TF), a member of the Snail family, serves as a master regulator of the gland-reconstituting activity of normal mammary stem cells, and that forced expression of Slug in collaboration with Sox9 in breast cancer cells can efficiently induce entrance into the TIC state. However, these earlier studies focused on xenograft models with cultured cell lines and involved ectopic expression of EMT-TFs, often at non-physiological levels. Using genetically engineered knock-in reporter mouse lines, here we show that normal gland-reconstituting mammary stem cells residing in the basal layer of the mammary epithelium and breast TICs originating in the luminal layer exploit the paralogous EMT-TFs Slug and Snail, respectively, which induce distinct EMT programs. Broadly, our findings suggest that the seemingly similar stem-cell programs operating in TICs and normal stem cells of the corresponding normal tissue are likely to differ significantly in their details.
Figures









References
-
- Al-Hajj M, Clarke MF. Self-renewal and solid tumor stem cells. Oncogene. 2004;23:7274–7282. doi:10.1038/sj.onc.1207947. - PubMed
-
- O'Brien CA, Kreso A, Dick JE. Cancer stem cells in solid tumors: an overview. Seminars in radiation oncology. 2009;19:71–77. doi:10.1016/j.semradonc.2008.11.001. - PubMed
-
- Visvader JE, Lindeman GJ. Cancer stem cells: current status and evolving complexities. Cell Stem Cell. 2012;10:717–728. doi:10.1016/j.stem.2012.05.007. - PubMed
-
- Gupta PB, et al. Stochastic state transitions give rise to phenotypic equilibrium in populations of cancer cells. Cell. 2011;146:633–644. doi:10.1016/j.cell.2011.07.026. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

