Severe acute respiratory syndrome coronavirus E protein transports calcium ions and activates the NLRP3 inflammasome
- PMID: 26331680
- PMCID: PMC4619128
- DOI: 10.1016/j.virol.2015.08.010
Severe acute respiratory syndrome coronavirus E protein transports calcium ions and activates the NLRP3 inflammasome
Abstract
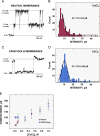
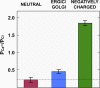
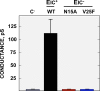
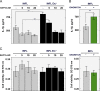
Severe acute respiratory syndrome coronavirus (SARS-CoV) envelope (E) protein is a viroporin involved in virulence. E protein ion channel (IC) activity is specifically correlated with enhanced pulmonary damage, edema accumulation and death. IL-1β driven proinflammation is associated with those pathological signatures, however its link to IC activity remains unknown. In this report, we demonstrate that SARS-CoV E protein forms protein-lipid channels in ERGIC/Golgi membranes that are permeable to calcium ions, a highly relevant feature never reported before. Calcium ions together with pH modulated E protein pore charge and selectivity. Interestingly, E protein IC activity boosted the activation of the NLRP3 inflammasome, leading to IL-1β overproduction. Calcium transport through the E protein IC was the main trigger of this process. These findings strikingly link SARS-CoV E protein IC induced ionic disturbances at the cell level to immunopathological consequences and disease worsening in the infected organism.
Keywords: Calcium; Coronavirus; E protein; Inflammasome; Ion channel; Pathogenesis; SARS-CoV; Viroporin.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures


References
-
- Annan A., Baldwin H.J., Corman V.M., Klose S.M., Owusu M., Nkrumah E.E., Badu E.K., Anti P., Agbenyega O., Meyer B., Oppong S., Sarkodie Y.A., Kalko E.K., Lina P.H., Godlevska E.V., Reusken C., Seebens A., Gloza-Rausch F., Vallo P., Tschapka M., Drosten C., Drexler J.F. Human betacoronavirus 2c EMC/2012-related viruses in Bats, Ghana and Europe. Emerg. Infect. Dis. 2013;19:456–459. - PMC - PubMed
-
- Campanella M., de Jong A.S., Lanke K.W., Melchers W.J., Willems P.H., Pinton P., Rizzuto R., van Kuppeveld F.J. The coxsackievirus 2B protein suppresses apoptotic host cell responses by manipulating intracellular Ca2+ homeostasis. J. Biol. Chem. 2004;279:18440–18450. - PubMed
-
- Chandra S., Kable E.P., Morrison G.H., Webb W.W. Calcium sequestration in the Golgi apparatus of cultured mammalian cells revealed by laser scanning confocal microscopy and ion microscopy. J. Cell Sci. 1991;100:747–752. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

