CAR-T Cell Therapy for Lymphoma
- PMID: 26332003
- PMCID: PMC4732525
- DOI: 10.1146/annurev-med-051914-021702
CAR-T Cell Therapy for Lymphoma
Abstract
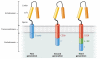
Lymphomas arise from clonal expansions of B, T, or NK cells at different stages of differentiation. Because they occur in the immunocyte-rich lymphoid tissues, they are easily accessible to antibodies and cell-based immunotherapy. Expressing chimeric antigen receptors (CARs) on T cells is a means of combining the antigen-binding site of a monoclonal antibody with the activating machinery of a T cell, enabling antigen recognition independent of major histocompatibility complex restriction, while retaining the desirable antitumor properties of a T cell. Here, we discuss the basic design of CARs and their potential advantages and disadvantages over other immune therapies for lymphomas. We review current clinical trials in the field and consider strategies to improve the in vivo function and safety of immune cells expressing CARs. The ultimate driver of CAR development and implementation for lymphoma will be the demonstration of their ability to safely and cost-effectively cure these malignancies.
Keywords: CD19; CD20; CD30; adoptive T cell therapy; immunotherapy; kappa light chain.
Figures
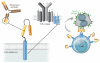
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

