Biogeography of Parasitic Nematode Communities in the Galápagos Giant Tortoise: Implications for Conservation Management
- PMID: 26332126
- PMCID: PMC4567182
- DOI: 10.1371/journal.pone.0135684
Biogeography of Parasitic Nematode Communities in the Galápagos Giant Tortoise: Implications for Conservation Management
Abstract
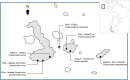
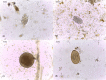
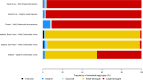
The Galápagos giant tortoise is an icon of the unique, endemic biodiversity of Galápagos, but little is known of its parasitic fauna. We assessed the diversity of parasitic nematode communities and their spatial distributions within four wild tortoise populations comprising three species across three Galápagos islands, and consider their implication for Galápagos tortoise conservation programmes. Coprological examinations revealed nematode eggs to be common, with more than 80% of tortoises infected within each wild population. Faecal samples from tortoises within captive breeding centres on Santa Cruz, Isabela and San Cristobal islands also were examined. Five different nematode egg types were identified: oxyuroid, ascarid, trichurid and two types of strongyle. Sequencing of the 18S small-subunit ribosomal RNA gene from adult nematodes passed with faeces identified novel sequences indicative of rhabditid and ascaridid species. In the wild, the composition of nematode communities varied according to tortoise species, which co-varied with island, but nematode diversity and abundance were reduced or altered in captive-reared animals. Evolutionary and ecological factors are likely responsible for the variation in nematode distributions in the wild. This possible species/island-parasite co-evolution has not been considered previously for Galápagos tortoises. We recommend that conservation efforts, such as the current Galápagos tortoise captive breeding/rearing and release programme, be managed with respect to parasite biogeography and host-parasite co-evolutionary processes in addition to the biogeography of the host.
Conflict of interest statement
Figures


References
-
- Quoy JRC, Gaimard JP. Description d'une nouvelle espèce de tortue et de trois espèces nouvelles de scinques. Bulletin des Sciences Naturelles et de Géologie. 1824;1:90–1.
-
- Turtle Taxonomy Working Group. Turtles of the World, 2012 Update: Annotated Checklist of Taxonomy, Synonymy, Distribution, and Conservation Status 2012.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

