Deferoxamine preconditioning to restore impaired HIF-1α-mediated angiogenic mechanisms in adipose-derived stem cells from STZ-induced type 1 diabetic rats
- PMID: 26332145
- PMCID: PMC6495947
- DOI: 10.1111/cpr.12209
Deferoxamine preconditioning to restore impaired HIF-1α-mediated angiogenic mechanisms in adipose-derived stem cells from STZ-induced type 1 diabetic rats
Abstract
Objectives: Both excessive and insufficient angiogenesis are associated with progression of diabetic complications, of which poor angiogenesis is an important feature. Currently, adipose-derived stem cells (ADSCs) are considered to be a promising source to aid therapeutic neovascularization. However, functionality of these cells is impaired by diabetes which can result from a defect in hypoxia-inducible factor-1 (HIF-1), a key mediator involved in neovascularization. In the current study, we sought to explore effectiveness of pharmacological priming with deferoxamine (DFO) as a hypoxia mimetic agent, to restore the compromised angiogenic pathway, with the aid of ADSCs derived from streptozotocin (STZ)-induced type 1 diabetic rats ('diabetic ADSCs').
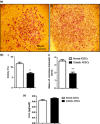
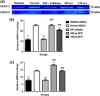
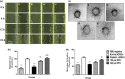
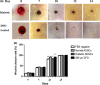
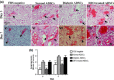
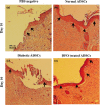
Materials and methods: Diabetic ADSCs were treated with DFO and compared to normal and non-treated diabetic ADSCs for expression of HIF-1α, VEGF, FGF-2 and SDF-1, at mRNA and protein levels, using qRT-PCR, western blotting and ELISA assay. Activity of matrix metalloproteinases -2 and -9 were measured using a gelatin zymography assay. Angiogenic potential of conditioned media derived from normal, DFO-treated and non-treated diabetic ADSCs were determined by in vitro (in HUVECs) and in vivo experiments including scratch assay, three-dimensional tube formation testing and surgical wound healing models.
Results: DFO remarkably enhanced expression of noted genes by mRNA and protein levels and restored activity of matrix metalloproteinases -2 and -9. Compromised angiogenic potential of conditioned medium derived from diabetic ADSCs was restored by DFO both in vitro and in vivo experiments.
Conclusion: DFO preconditioning restored neovascularization potential of ADSCs derived from diabetic rats by affecting the HIF-1α pathway.
© 2015 John Wiley & Sons Ltd.
Conflict of interest statement
The authors confirm that there are no conflicts of interest.
Figures





Similar articles
-
Deferoxamine enhances neovascularization and accelerates wound healing in diabetic rats via the accumulation of hypoxia-inducible factor-1α.Diabetes Res Clin Pract. 2013 Jul;101(1):62-71. doi: 10.1016/j.diabres.2013.04.012. Epub 2013 May 28. Diabetes Res Clin Pract. 2013. PMID: 23726275
-
Restoring the Angiogenic Capacity of the Human Diabetic Adipose-derived mesenchymal stem cells Primed with Deferoxamine as a Hypoxia Mimetic Agent: Role of HIF-1α.Adv Pharm Bull. 2023 Mar;13(2):350-360. doi: 10.34172/apb.2023.021. Epub 2022 Jan 10. Adv Pharm Bull. 2023. PMID: 37342375 Free PMC article.
-
Deferoxamine preconditioning potentiates mesenchymal stem cell homing in vitro and in streptozotocin-diabetic rats.Expert Opin Biol Ther. 2013 Jul;13(7):959-72. doi: 10.1517/14712598.2013.782390. Epub 2013 Mar 28. Expert Opin Biol Ther. 2013. PMID: 23536977
-
Hypoxia inducible factor-1 alpha, endothelial progenitor cells, monocytes, cardiovascular risk, wound healing, cobalt and hydralazine: a unifying hypothesis.Curr Drug Targets. 2008 May;9(5):422-35. doi: 10.2174/138945008784221215. Curr Drug Targets. 2008. PMID: 18473772 Review.
-
Deferoxamine: An Angiogenic and Antioxidant Molecule for Tissue Regeneration.Tissue Eng Part B Rev. 2019 Dec;25(6):461-470. doi: 10.1089/ten.TEB.2019.0111. Epub 2019 Sep 11. Tissue Eng Part B Rev. 2019. PMID: 31184273 Review.
Cited by
-
Secretome from human adipose-derived mesenchymal stem cells promotes blood vessel formation and pericyte coverage in experimental skin repair.PLoS One. 2022 Dec 19;17(12):e0277863. doi: 10.1371/journal.pone.0277863. eCollection 2022. PLoS One. 2022. PMID: 36534643 Free PMC article.
-
Impact of insulin producing cells derived from adipose tissue mesenchymal stem cells on testicular dysfunction of diabetic rats.Heliyon. 2021 Nov 1;7(11):e08316. doi: 10.1016/j.heliyon.2021.e08316. eCollection 2021 Nov. Heliyon. 2021. PMID: 34820536 Free PMC article.
-
Boosting therapeutic efficacy of mesenchymal stem cells in pulmonary fibrosis: The role of genetic modification and preconditioning strategies.Iran J Basic Med Sci. 2023;26(9):1001-1015. doi: 10.22038/IJBMS.2023.69023.15049. Iran J Basic Med Sci. 2023. PMID: 37605719 Free PMC article. Review.
-
Regenerative Medicine and Angiogenesis; Challenges and Opportunities.Adv Pharm Bull. 2020 Sep;10(4):490-501. doi: 10.34172/apb.2020.061. Epub 2020 Aug 9. Adv Pharm Bull. 2020. PMID: 33072530 Free PMC article. Review.
-
Chelating the valley of death: Deferoxamine's path from bench to wound clinic.Front Med (Lausanne). 2023 Feb 15;10:1015711. doi: 10.3389/fmed.2023.1015711. eCollection 2023. Front Med (Lausanne). 2023. PMID: 36873870 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

