Acceptability and Accuracy of Cervical Cancer Screening Using a Self-Collected Tampon for HPV Messenger-RNA Testing among HIV-Infected Women in South Africa
- PMID: 26332236
- PMCID: PMC4557833
- DOI: 10.1371/journal.pone.0137299
Acceptability and Accuracy of Cervical Cancer Screening Using a Self-Collected Tampon for HPV Messenger-RNA Testing among HIV-Infected Women in South Africa
Abstract
Background: HIV increases women's risk for high-risk human papillomavirus (hrHPV) infection and invasive cervical cancer. South Africa has a high HIV prevalence but low cervical cancer screening coverage. Self-collection of cervical specimens and hrHPV testing, including hrHPV messenger-RNA (mRNA) testing, are methods aimed at increasing screening rates. However, data are limited on the acceptability and accuracy of tampon-based self-collection for hrHPV mRNA testing in HIV-infected women.
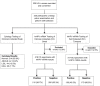
Methods: We recruited 325 HIV-infected women seeking care at a government HIV clinic in Pretoria, South Africa. A clinician performed a pelvic examination and obtained an endocervical specimen. Study participants performed self-collection using a tampon. Both clinician- and self-collected specimens were tested for hrHPV mRNA. Acceptability of both collection methods was assessed, the prevalence of hrHPV mRNA in our study population was estimated, test positivity of the two collection methods were compared, and test agreement was assessed by calculating the κ-statistic, sensitivity, and specificity.
Results: Over 90% of women reported no difficulties self-collecting specimens and 82% were willing to perform the tampon-collection at home. Based on clinician-collection specimens, the prevalence of hrHPV mRNA in our study population was 36.7% (95% CI: 31.4%- 42.0%). There was no difference in test positivity between clinician-collection, 36.7%, and tampon-collection, 43.5% (p-value = 0.08). Using clinician-collection as the reference test, the sensitivity and specificity for hrHPV mRNA of tampon-collection were 77.4% (95% CI: 69.8-85.0%) and 77.8% (95% CI: 71.9-83.6%), respectively.
Conclusions: Tampon-based self-collection is acceptable to women and has similar hrHPV mRNA positivity rates as clinician-collection, but has reduced sensitivity and specificity compared to clinician-collection. The hrHPV mRNA prevalence in our study population is high, but similar to other high-risk populations, and highlights the need for improved cervical cancer screening. Further research into the optimal use of tampon-based collection as a cervical cancer screening tool is warranted.
Conflict of interest statement
Figures
References
-
- Soerjomataram FJ, Ervik I, Dikshit M. R. Eser, S. Mathers, C. Rebelo, M. Parkin, DM. Forman, D. Bray, F. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. 2013. - PubMed
-
- UNAIDS. AIDSinfo Country fact sheet—South Africa. 2013.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical