A positive family history as a risk factor for prostate cancer in a population-based study with organised prostate-specific antigen screening: results of the Swiss European Randomised Study of Screening for Prostate Cancer (ERSPC, Aarau)
- PMID: 26332304
- PMCID: PMC4955666
- DOI: 10.1111/bju.13310
A positive family history as a risk factor for prostate cancer in a population-based study with organised prostate-specific antigen screening: results of the Swiss European Randomised Study of Screening for Prostate Cancer (ERSPC, Aarau)
Abstract
Objective: To assess the value of a positive family history (FH) as a risk factor for prostate cancer incidence and grade among men undergoing organised prostate-specific antigen (PSA) screening in a population-based study.
Subjects and methods: The study cohort comprised all attendees of the Swiss arm of the European Randomised Study of Screening for Prostate Cancer (ERSPC) with systematic PSA level tests every 4 years. Men reporting first-degree relative(s) diagnosed with prostate cancer were considered to have a positive FH. Biopsy was exclusively PSA triggered at a PSA level threshold of 3 ng/mL. The primary endpoint was prostate cancer diagnosis. Kaplan-Meier and Cox regression analyses were used.
Results: Of 4 932 attendees with a median (interquartile range, IQR) age of 60.9 (57.6-65.1) years, 334 (6.8%) reported a positive FH. The median (IQR) follow-up duration was 11.6 (10.3-13.3) years. Cumulative prostate cancer incidence was 60/334 (18%, positive FH) and 550/4 598 (12%, negative FH) [odds ratio 1.6, 95% confidence interval (CI) 1.2-2.2, P = 0.001). In both groups, most prostate cancer diagnosed was low grade. There were no significant differences in PSA level at diagnosis, biopsy Gleason score or Gleason score on pathological specimen among men who underwent radical prostatectomy between both groups. On multivariable analysis, age (hazard ratio [HR] 1.04, 95% CI 1.02-1.06), baseline PSA level (HR 1.13, 95% CI 1.12-1.14), and FH (HR 1.6, 95% CI 1.24-2.14) were independent predictors for overall prostate cancer incidence (all P < 0.001). Only baseline PSA level (HR 1.14, 95% CI 1.12-1.16, P < 0.001) was an independent predictor of Gleason score ≥7 prostate cancer on prostate biopsy. The proportion of interval prostate cancer diagnosed in-between the screening rounds was not significantly different.
Conclusion: Irrespective of the FH status, the current PSA-based screening setting detects the majority of aggressive prostate cancers and missed only a minority of interval cancers with a 4-year screening algorithm. Our results suggest that men with a positive FH are at increased risk of low-grade but not aggressive prostate cancer.
Keywords: positive family history; prostate cancer aggressiveness; prostate cancer screening; prostate-specific antigen; screening intensity.
© 2015 The Authors BJU International © 2015 BJU International Published by John Wiley & Sons Ltd.
Figures
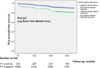

Comment in
-
To PSA or not to PSA? Still a question for men with a family history of prostate cancer.BJU Int. 2016 Apr;117(4):545. doi: 10.1111/bju.13372. BJU Int. 2016. PMID: 26969030 No abstract available.
References
-
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA: a cancer journal for clinicians. 2014 Jan-Feb;64:9–29. - PubMed
-
- Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. European journal of cancer. 2010 Mar;46:765–781. - PubMed
-
- Draisma G, Boer R, Otto SJ, et al. Lead times and overdetection due to prostate-specific antigen screening: estimates from the European Randomized Study of Screening for Prostate Cancer. Journal of the National Cancer Institute. 2003 Jun 18;95:868–878. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

