Chemical Tools To Decipher Regulation of Phosphatases by Proline Isomerization on Eukaryotic RNA Polymerase II
- PMID: 26332362
- PMCID: PMC4788383
- DOI: 10.1021/acschembio.5b00296
Chemical Tools To Decipher Regulation of Phosphatases by Proline Isomerization on Eukaryotic RNA Polymerase II
Abstract
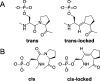
Proline isomerization greatly impacts biological signaling but is subtle and difficult to detect in proteins. We characterize this poorly understood regulatory mechanism for RNA polymerase II carboxyl terminal domain (CTD) phosphorylation state using novel, direct, and quantitative chemical tools. We determine the proline isomeric preference of three CTD phosphatases: Ssu72 as cis-proline specific, Scp1 and Fcp1 as strongly trans-preferred. Due to this inherent characteristic, these phosphatases respond differently to enzymes that catalyze the isomerization of proline, like Ess1/Pin1. We demonstrate that this selective regulation of RNA polymerase II phosphorylation state exists within human cells, consistent with in vitro assays. These results support a model in which, instead of a global enhancement of downstream enzymatic activities, proline isomerases selectively boost the activity of a subset of CTD regulatory factors specific for cis-proline. This leads to diversified phosphorylation states of CTD in vitro and in cells. We provide the chemical tools to investigate proline isomerization and its ability to selectively enhance signaling in transcription and other biological contexts.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





Similar articles
-
cis-Proline-mediated Ser(P)5 dephosphorylation by the RNA polymerase II C-terminal domain phosphatase Ssu72.J Biol Chem. 2011 Feb 18;286(7):5717-26. doi: 10.1074/jbc.M110.197129. Epub 2010 Dec 15. J Biol Chem. 2011. PMID: 21159777 Free PMC article.
-
Chemical Tools for Studying the Impact of cis/trans Prolyl Isomerization on Signaling: A Case Study on RNA Polymerase II Phosphatase Activity and Specificity.Methods Enzymol. 2018;607:269-297. doi: 10.1016/bs.mie.2018.04.020. Epub 2018 Jun 23. Methods Enzymol. 2018. PMID: 30149861 Free PMC article.
-
Pin1 modulates the dephosphorylation of the RNA polymerase II C-terminal domain by yeast Fcp1.FEBS Lett. 2002 Feb 27;513(2-3):305-11. doi: 10.1016/s0014-5793(02)02288-3. FEBS Lett. 2002. PMID: 11904169
-
The diverse roles of RNA polymerase II C-terminal domain phosphatase SCP1.BMB Rep. 2014 Apr;47(4):192-6. doi: 10.5483/bmbrep.2014.47.4.060. BMB Rep. 2014. PMID: 24755554 Free PMC article. Review.
-
A structural perspective of CTD function.Genes Dev. 2005 Jun 15;19(12):1401-15. doi: 10.1101/gad.1318105. Genes Dev. 2005. PMID: 15964991 Review.
Cited by
-
Phosphatase activity of small C-terminal domain phosphatase 1 (SCP1) controls the stability of the key neuronal regulator RE1-silencing transcription factor (REST).J Biol Chem. 2018 Oct 26;293(43):16851-16861. doi: 10.1074/jbc.RA118.004722. Epub 2018 Sep 14. J Biol Chem. 2018. PMID: 30217818 Free PMC article.
-
Tracking Inhibition of Human Small C-Terminal Domain Phosphatase 1 Using 193 nm Ultraviolet Photodissociation Mass Spectrometry.J Am Soc Mass Spectrom. 2024 Jun 5;35(6):1330-1341. doi: 10.1021/jasms.4c00098. Epub 2024 Apr 25. J Am Soc Mass Spectrom. 2024. PMID: 38662915 Free PMC article.
-
Thr4 phosphorylation on RNA Pol II occurs at early transcription regulating 3'-end processing.Sci Adv. 2024 Sep 6;10(36):eadq0350. doi: 10.1126/sciadv.adq0350. Epub 2024 Sep 6. Sci Adv. 2024. PMID: 39241064 Free PMC article.
-
Targeting Hepatitis B Virus Covalently Closed Circular DNA and Hepatitis B Virus X Protein: Recent Advances and New Approaches.ACS Infect Dis. 2019 Oct 11;5(10):1657-1667. doi: 10.1021/acsinfecdis.9b00249. Epub 2019 Sep 27. ACS Infect Dis. 2019. PMID: 31525994 Free PMC article.
-
Novel polysaccharide binding to the N-terminal tail of galectin-3 is likely modulated by proline isomerization.Glycobiology. 2017 Nov 1;27(11):1038-1051. doi: 10.1093/glycob/cwx071. Glycobiology. 2017. PMID: 28973299 Free PMC article.
References
-
- Eick D, Geyer M. The RNA polymerase II carboxyterminal domain (CTD) code. Chem Rev (Washington, DC, US) 2013;113:8456–8490. - PubMed
-
- Bataille AR, Jeronimo C, Jacques PE, Laramee L, Fortin ME, Forest A, Bergeron M, Hanes SD, Robert F. A universal RNA polymerase II CTD cycle is orchestrated by complex interplays between kinase, phosphatase, and isomerase enzymes along genes. Mol Cell. 2012;45:158–170. - PubMed
-
- Schroder S, Herker E, Itzen F, He D, Thomas S, Gilchrist DA, Kaehlcke K, Cho S, Pollard KS, Capra JA, Schnolzer M, Cole PA, Geyer M, Bruneau BG, Adelman K, Ott M. Acetylation of RNA polymerase II regulates growth-factor-induced gene transcription in mammalian cells. Mol Cell. 2013;52:314–324. - PMC - PubMed
-
- Conaway JW, Shilatifard A, Dvir A, Conaway RC. Control of elongation by RNA polymerase II. Trends Biochem Sci. 2000;25:375–380. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

