Coordination Chemistry of Microbial Iron Transport
- PMID: 26332443
- PMCID: PMC4576731
- DOI: 10.1021/acs.accounts.5b00301
Coordination Chemistry of Microbial Iron Transport
Abstract
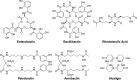
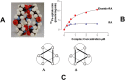
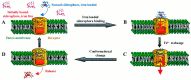
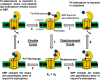
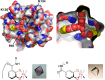
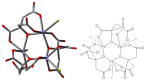
This Account focuses on the coordination chemistry of the microbial iron chelators called siderophores. The initial research (early 1970s) focused on simple analogs of siderophores, which included hydroxamate, catecholate, or hydroxycarboxylate ligands. The subsequent work increasingly focused on the transport of siderophores and their microbial iron transport. Since these are pseudo-octahedral complexes often composed of bidentate ligands, there is chirality at the metal center that in principle is independent of the ligand chirality. It has been shown in many cases that chiral recognition of the complex occurs. Many techniques have been used to elucidate the iron uptake processes in both Gram-positive (single membrane) and Gram-negative (double membrane) bacteria. These have included the use of radioactive labels (of ligand, metal, or both), kinetically inert metal complexes, and Mössbauer spectroscopy. In general, siderophore recognition and transport involves receptors that recognize the metal chelate portion of the iron-siderophore complex. A second, to date less commonly found, mechanism called the siderophore shuttle involves the receptor binding an apo-siderophore. Since one of the primary ways that microbes compete with each other for iron stores is the strength of their competing siderophore complexes, it became important early on to characterize the solution thermodynamics of these species. Since the acidity of siderophores varies significantly, just the stability constant does not give a direct measure of the relative competitive strength of binding. For this reason, the pM value is compared. The pM, like pH, is a measure of the negative log of the free metal ion concentration, typically calculated at pH 7.4, and standard total concentrations of metal and ligand. The characterization of the electronic structure of ferric siderophores has done much to help explain the high stability of these complexes. A new chapter in siderophore science has emerged with the characterization of what are now called siderocalins. Initially found as a protein of the human innate immune system, these proteins bind both ferric and apo-siderophores to inactivate the siderophore transport system and hence deny iron to an invading pathogenic microbe. Siderocalins also can play a role in iron transport of the host, particularly in the early stages of fetal development. Finally, it is speculated that the molecular targets of siderocalins in different species differ based on the siderophore structures of the most important bacterial pathogens of those species.
Figures
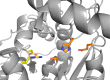

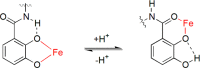

References
-
- Stintzi A.; Raymond K. N.. Siderophore Chemistry. In Molecular and Cellular Iron Transport; Templeton D. E., Ed.; Marcel Dekker, Inc.: New York, 2001; pp 273–319.
-
- Raymond K. N.; Dertz E. A.. Siderophores and Transferrins. In Comprehensive Coordination Chemistry II; Que L. Jr., Tolman W. B., Eds.; Elsevier, Ltd.: San Diego, CA, 2003; Vol. 8, pp 141–168.
-
- Dertz E. A.; Raymond K. N.. Biochemical and Physical Properties of Siderophores. In Iron Transport in Bacteria; Crosa J., Mey A. R., Payne S., Eds.; ASM Press: Washington, DC, 2004; pp 3–16.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

