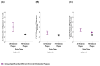Oligomeric α-synuclein and β-amyloid variants as potential biomarkers for Parkinson's and Alzheimer's diseases
- PMID: 26332448
- PMCID: PMC4718789
- DOI: 10.1111/ejn.13056
Oligomeric α-synuclein and β-amyloid variants as potential biomarkers for Parkinson's and Alzheimer's diseases
Abstract
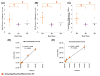
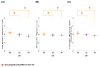
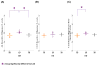
Oligomeric forms of α-synuclein and β-amyloid are toxic protein variants that are thought to contribute to the onset and progression of Parkinson's disease (PD) and Alzheimer's disease (AD), respectively. The detection of toxic variants in human cerebrospinal fluid (CSF) and blood has great promise for facilitating early and accurate diagnoses of these devastating diseases. Two hurdles that have impeded the use of these protein variants as biomarkers are the availability of reagents that can bind the different variants and a sensitive assay to detect their very low concentrations. We previously isolated antibody-based reagents that selectively bind two different oligomeric variants of α-synuclein and two of β-amyloid, and developed a phage-based capture enzyme-linked immunosorbent assay (ELISA) with subfemtomolar sensitivity to quantify their presence. Here, we used these reagents to show that these oligomeric α-synuclein variants are preferentially present in PD brain tissue, CSF and serum, and that the oligomeric β-amyloid variants are preferentially present in AD brain tissue, CSF, and serum. Some AD samples also had α-synuclein pathology and some PD samples also had β-amyloid pathology, and, very intriguingly, these PD cases also had a history of dementia. Detection of different oligomeric α-synuclein and β-amyloid species is an effective method for identifying tissue, CSF and sera from PD and AD samples, respectively, and samples that also contained early stages of other protein pathologies, indicating their potential value as blood-based biomarkers for neurodegenerative diseases.
Keywords: brain tissue; cerebrospinal fluid; phage capture enzyme-linked immunosorbent assay; serum; single-chain variable fragments.
© 2015 Federation of European Neuroscience Societies and John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures
Comment in
-
Can pathological oligomeric proteins make good biomarkers? (Commentary on Williams et al.).Eur J Neurosci. 2016 Jan;43(1):1-2. doi: 10.1111/ejn.13115. Epub 2015 Dec 10. Eur J Neurosci. 2016. PMID: 26503665 No abstract available.
References
-
- Beach TG, Adler CH, Lue L, Sue LI, Bachalakuri J, Henry-Watson J, Sasse J, Boyer S, Shirohi S, Brooks R, Eschbacher J, White CL, 3rd, Akiyama H, Caviness J, Shill HA, Connor DJ, Sabbagh MN, Walker DG. Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol. 2009;117:613–634. - PMC - PubMed
-
- Beach TG, Adler CH, Sue LI, Serrano G, Shill HA, Walker DG, Lue L, Roher AE, Dugger BN, Maarouf C, Birdsill AC, Intorcia A, Saxon-Labelle M, Pullen J, Scroggins A, Filon J, Scott S, Hoffman B, Garcia A, Caviness JN, Hentz JG, Driver-Dunckley E, Jacobson SA, Davis KJ, Belden CM, Long KE, Malek-Ahmadi M, Powell JJ, Gale LD, Nicholson LR, Caselli RJ, Woodruff BK, Rapscak SZ, Ahern GL, Shi J, Burke AD, Reiman EM, Sabbagh MN. Arizona Study of Aging and Neurodegenerative Disorders and Brain and Body Donation Program. Neuropathology. 2015;35:354–389. - PMC - PubMed
-
- Chaari A, Hoarau-Véchot J, Ladjimi M. Applying chaperones to protein-misfolding disorders: Molecular chaperones against α-synuclein in Parkinson’s disease. Int J Biol Macromol. 2013;60:196–205. - PubMed
-
- Cox D, Carver JA, Ecroyd H. Preventing α-synuclein aggregation: The role of the small heat-shock molecular chaperone proteins. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2014;1842:1830–1843. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical