Altered FGF Signaling Pathways Impair Cell Proliferation and Elevation of Palate Shelves
- PMID: 26332583
- PMCID: PMC4558018
- DOI: 10.1371/journal.pone.0136951
Altered FGF Signaling Pathways Impair Cell Proliferation and Elevation of Palate Shelves
Abstract
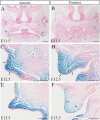
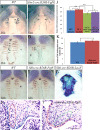
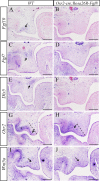
In palatogenesis, palatal shelves are patterned along the mediolateral axis as well as the anteroposterior axis before the onset of palatal fusion. Fgf10 specifically expressed in lateral mesenchyme of palate maintains Shh transcription in lateral epithelium, while Fgf7 activated in medial mesenchyme by Dlx5, suppressed the expansion of Shh expression to medial epithelium. How FGF signaling pathways regulate the cell behaviors of developing palate remains elusive. In our study, we found that when Fgf8 is ectopically expressed in the embryonic palatal mesenchyme, the elevation of palatal shelves is impaired and the posterior palatal shelves are enlarged, especially in the medial side. The palatal deformity results from the drastic increase of cell proliferation in posterior mesenchyme and decrease of cell proliferation in epithelium. The expression of mesenchymal Fgf10 and epithelial Shh in the lateral palate, as well as the Dlx5 and Fgf7 transcription in the medial mesenchyme are all interrupted, indicating that the epithelial-mesenchymal interactions during palatogenesis are disrupted by the ectopic activation of mesenchymal Fgf8. Besides the altered Fgf7, Fgf10, Dlx5 and Shh expression pattern, the reduced Osr2 expression domain in the lateral mesenchyme also suggests an impaired mediolateral patterning of posterior palate. Moreover, the ectopic Fgf8 expression up-regulates pJak1 throughout the palatal mesenchyme and pErk in the medial mesenchyme, but down-regulates pJak2 in the epithelium, suggesting that during normal palatogenesis, the medial mesenchymal cell proliferation is stimulated by FGF/Erk pathway, while the epithelial cell proliferation is maintained through FGF/Jak2 pathway.
Conflict of interest statement
Figures



Similar articles
-
Indirect modulation of Shh signaling by Dlx5 affects the oral-nasal patterning of palate and rescues cleft palate in Msx1-null mice.Development. 2009 Dec;136(24):4225-33. doi: 10.1242/dev.036723. Development. 2009. PMID: 19934017 Free PMC article.
-
Pax9 regulates a molecular network involving Bmp4, Fgf10, Shh signaling and the Osr2 transcription factor to control palate morphogenesis.Development. 2013 Dec;140(23):4709-18. doi: 10.1242/dev.099028. Epub 2013 Oct 30. Development. 2013. PMID: 24173808 Free PMC article.
-
A Shh-Foxf-Fgf18-Shh Molecular Circuit Regulating Palate Development.PLoS Genet. 2016 Jan 8;12(1):e1005769. doi: 10.1371/journal.pgen.1005769. eCollection 2016 Jan. PLoS Genet. 2016. PMID: 26745863 Free PMC article.
-
The Function and Regulatory Network of Pax9 Gene in Palate Development.J Dent Res. 2019 Mar;98(3):277-287. doi: 10.1177/0022034518811861. Epub 2018 Dec 24. J Dent Res. 2019. PMID: 30583699 Review.
-
Hedgehog signalling in development of the secondary palate.Front Oral Biol. 2012;16:52-9. doi: 10.1159/000337543. Epub 2012 Jun 25. Front Oral Biol. 2012. PMID: 22759669 Review.
Cited by
-
The Fibroblast Growth Factor 9 (Fgf9) Participates in Palatogenesis by Promoting Palatal Growth and Elevation.Front Physiol. 2021 Apr 20;12:653040. doi: 10.3389/fphys.2021.653040. eCollection 2021. Front Physiol. 2021. PMID: 33959039 Free PMC article.
-
FGF8 Signaling Alters the Osteogenic Cell Fate in the Hard Palate.J Dent Res. 2018 May;97(5):589-596. doi: 10.1177/0022034517750141. Epub 2018 Jan 17. J Dent Res. 2018. PMID: 29342370 Free PMC article.
-
Gene-Sex Interaction in Non-Syndromic Orofacial Cleft Subtypes: A Case-Control Study Among the Vietnamese Population.Genes (Basel). 2025 Jul 22;16(8):853. doi: 10.3390/genes16080853. Genes (Basel). 2025. PMID: 40869901 Free PMC article.
-
Determinants of orofacial clefting I: Effects of 5-Aza-2'-deoxycytidine on cellular processes and gene expression during development of the first branchial arch.Reprod Toxicol. 2017 Jan;67:85-99. doi: 10.1016/j.reprotox.2016.11.016. Epub 2016 Nov 30. Reprod Toxicol. 2017. PMID: 27915011 Free PMC article.
-
Disrupted tenogenesis in masseter as a potential cause of micrognathia.Int J Oral Sci. 2022 Oct 18;14(1):50. doi: 10.1038/s41368-022-00196-y. Int J Oral Sci. 2022. PMID: 36257937 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

