Safety, pharmacokinetics and neutralization of the broadly neutralizing HIV-1 human monoclonal antibody VRC01 in healthy adults
- PMID: 26332605
- PMCID: PMC4636891
- DOI: 10.1111/cei.12692
Safety, pharmacokinetics and neutralization of the broadly neutralizing HIV-1 human monoclonal antibody VRC01 in healthy adults
Abstract
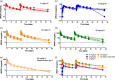
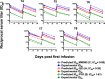
VRC-HIVMAB060-00-AB (VRC01) is a broadly neutralizing HIV-1 monoclonal antibody (mAb) isolated from the B cells of an HIV-infected patient. It is directed against the HIV-1 CD4 binding site and is capable of potently neutralizing the majority of diverse HIV-1 strains. This Phase I dose-escalation study in healthy adults was conducted at the National Institutes of Health (NIH) Clinical Center (Bethesda, MD, USA). Primary objectives were the safety, tolerability and pharmacokinetics (PK) of VRC01 intravenous (i.v.) infusion at 5, 20 or 40 mg/kg, given either once (20 mg/kg) or twice 28 days apart (all doses), and of subcutaneous (s.c.) delivery at 5 mg/kg compared to s.c. placebo given twice, 28 days apart. Cumulatively, 28 subjects received 43 VRC01 and nine received placebo administrations. There were no serious adverse events or dose-limiting toxicities. Mean 28-day serum trough concentrations after the first infusion were 35 and 57 μg/ml for groups infused with 20 mg/kg (n = 8) and 40 mg/kg (n = 5) doses, respectively. Mean 28-day trough concentrations after the second infusion were 56 and 89 μg/ml for the same two doses. Over the 5-40 mg/kg i.v. dose range (n = 18), the clearance was 0.016 l/h and terminal half-life was 15 days. After infusion VRC01 retained expected neutralizing activity in serum, and anti-VRC01 antibody responses were not detected. The human monoclonal antibody (mAb) VRC01 was well tolerated when delivered i.v. or s.c. The mAb demonstrated expected half-life and pharmacokinetics for a human immunoglobulin G. The safety and PK results support and inform VRC01 dosing schedules for planning HIV-1 prevention efficacy studies.
Trial registration: ClinicalTrials.gov NCT01993706.
Keywords: HIV-1; Phase I clinical trial; monoclonal antibody; passive immunization; pharmacokinetics.
© 2015 British Society for Immunology.
Figures


References
-
- Mascola JR, Montefiori DC. The role of antibodies in HIV vaccines. Annu Rev Immunol. 2010;28:413–44. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

