Selection of recombinant anti-SH3 domain antibodies by high-throughput phage display
- PMID: 26332758
- PMCID: PMC4622221
- DOI: 10.1002/pro.2799
Selection of recombinant anti-SH3 domain antibodies by high-throughput phage display
Abstract
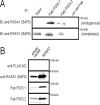
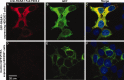
Antibodies are indispensable tools in biochemical research and play an expanding role as therapeutics. While hybridoma technology is the dominant method for antibody production, phage display is an emerging technology. Here, we developed and employed a high-throughput pipeline that enables selection of antibodies against hundreds of antigens in parallel. Binding selections using a phage-displayed synthetic antigen-binding fragment (Fab) library against 110 human SH3 domains yielded hundreds of Fabs targeting 58 antigens. Affinity assays demonstrated that representative Fabs bind tightly and specifically to their targets. Furthermore, we developed an efficient affinity maturation strategy adaptable to high-throughput, which increased affinity dramatically but did not compromise specificity. Finally, we tested Fabs in common cell biology applications and confirmed recognition of the full-length antigen in immunoprecipitation, immunoblotting and immunofluorescence assays. In summary, we have established a rapid and robust high-throughput methodology that can be applied to generate highly functional and renewable antibodies targeting protein domains on a proteome-wide scale.
Keywords: SH3 domain; antibodies; high throughput method; phage display.
© 2015 The Protein Society.
Figures


References
-
- Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. - PubMed
-
- Dubel S, Stoevesandt O, Taussig MJ, Hust M. Generating recombinant antibodies to the complete human proteome. Trends Biotechnol. 2010;28:333–339. - PubMed
-
- Sidhu SS. Antibodies for all: the case for genome-wide affinity reagents. FEBS Lett. 2012;586:2778–2779. - PubMed
-
- McCafferty J, Griffiths AD, Winter G, Chiswell DJ. Phage antibodies: filamentous phage displaying antibody variable domains. Nature. 1990;348:552–554. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

