Membrane lipid rafts and neurobiology: age-related changes in membrane lipids and loss of neuronal function
- PMID: 26332795
- PMCID: PMC4983616
- DOI: 10.1113/JP270590
Membrane lipid rafts and neurobiology: age-related changes in membrane lipids and loss of neuronal function
Abstract
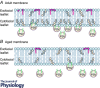
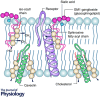
A better understanding of the cellular physiological role that plasma membrane lipids, fatty acids and sterols play in various cellular systems may yield more insight into how cellular and whole organ function is altered during the ageing process. Membrane lipid rafts (MLRs) within the plasma membrane of most cells serve as key organizers of intracellular signalling and tethering points of cytoskeletal components. MLRs are plasmalemmal microdomains enriched in sphingolipids, cholesterol and scaffolding proteins; they serve as a platform for signal transduction, cytoskeletal organization and vesicular trafficking. Within MLRs are the scaffolding and cholesterol binding proteins named caveolin (Cav). Cavs not only organize a multitude of receptors including neurotransmitter receptors (NMDA and AMPA receptors), signalling proteins that regulate the production of cAMP (G protein-coupled receptors, adenylyl cyclases, phosphodiesterases (PDEs)), and receptor tyrosine kinases involved in growth (Trk), but also interact with components that modulate actin and tubulin cytoskeletal dynamics (e.g. RhoGTPases and actin binding proteins). MLRs are essential for the regulation of the physiology of organs such as the brain, and age-related loss of cholesterol from the plasma membrane leads to loss of MLRs, decreased presynaptic vesicle fusion, and changes in neurotransmitter release, all of which contribute to different forms of neurodegeneration. Thus, MLRs provide an active membrane domain that tethers and reorganizes the cytoskeletal machinery necessary for membrane and cellular repair, and genetic interventions that restore MLRs to normal cellular levels may be exploited as potential therapeutic means to reverse the ageing and neurodegenerative processes.
Published 2015. This article is a U.S. Government work and is in the public domain in the USA.
Figures

References
-
- Allen JA, Halverson‐Tamboli RA & Rasenick MM (2007). Lipid raft microdomains and neurotransmitter signalling. Nat Rev Neurosci 8, 128–140. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

