Mapping the electrophysiological and morphological properties of CA1 pyramidal neurons along the longitudinal hippocampal axis
- PMID: 26333017
- PMCID: PMC4760884
- DOI: 10.1002/hipo.22526
Mapping the electrophysiological and morphological properties of CA1 pyramidal neurons along the longitudinal hippocampal axis
Abstract
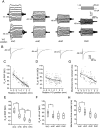
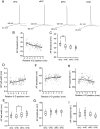
Differences in behavioral roles, anatomical connectivity, and gene expression patterns in the dorsal, intermediate, and ventral regions of the hippocampus are well characterized. Relatively fewer studies have, however, focused on comparing the physiological properties of neurons located at different dorsoventral extents of the hippocampus. Recently, we reported that dorsal CA1 neurons are less excitable than ventral neurons. There is little or no information for how neurons in the intermediate hippocampus compare to those from the dorsal and ventral ends. Also, it is not known whether the transition of properties along the dorsoventral axis is gradual or segmented. In this study, we developed a statistical model to predict the dorsoventral position of transverse hippocampal slices. Using current clamp recordings combined with this model, we found that CA1 neurons in dorsal, intermediate, and ventral hippocampus have distinct electrophysiological and morphological properties and that the transition in most (but not all) of these properties from the ventral to dorsal end is gradual. Using linear and segmented regression analyses, we found that input resistance and resting membrane potential changed linearly along the V-D axis. Interestingly, the transition in resonance frequency, rebound slope, dendritic branching in stratum radiatum, and action potential properties was segmented along the V-D axis. Together, the findings from this study highlight the heterogeneity in CA1 neuronal properties along the entire longitudinal axis of hippocampus.
Keywords: dendritic branching; dorsal hippocampus; firing output; intermediate hippocampus; intrinsic excitability; resonance frequency; ventral hippocampus.
© 2015 Wiley Periodicals, Inc.
Conflict of interest statement
Figures






References
-
- Akaikea K, Tanakaa S, Tojoa H, Fukumotoa SI, Imamura BSI, Takigawa M. Kainic acid-induced dorsal and ventral hippocampal seizures in rats. Brain Research. 2001;900:65–71. - PubMed
-
- Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: A review of anatomical data. Neuroscience. 1989;31:571–591. - PubMed
-
- Ashton D, Van Reempts J, Haseldonckx M, Willems R. Dorsal-ventral gradient in vulnerability of CA1 hippocampus to ischemia: a combined histological and electrophysiological study. Brain Research. 1989;487:368–372. - PubMed
-
- Bannerman DM, Rawlins JNP, McHugh SB, Deacon RMJ, Yee BK, Bast T, Zhang WN, Pothuizen HHJ, Feldon J. Regional dissociations within the hippocampus—memory and anxiety. Neuroscience & Biobehavioral Reviews. 2004;28:273–283. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

