Fluorescence In Situ Hybridization (FISH) Assays for Diagnosing Malaria in Endemic Areas
- PMID: 26333092
- PMCID: PMC4558036
- DOI: 10.1371/journal.pone.0136726
Fluorescence In Situ Hybridization (FISH) Assays for Diagnosing Malaria in Endemic Areas
Abstract
Malaria is a responsible for approximately 600 thousand deaths worldwide every year. Appropriate and timely treatment of malaria can prevent deaths but is dependent on accurate and rapid diagnosis of the infection. Currently, microscopic examination of the Giemsa stained blood smears is the method of choice for diagnosing malaria. Although it has limited sensitivity and specificity in field conditions, it still remains the gold standard for the diagnosis of malaria. Here, we report the development of a fluorescence in situ hybridization (FISH) based method for detecting malaria infection in blood smears and describe the use of an LED light source that makes the method suitable for use in resource-limited malaria endemic countries. The Plasmodium Genus (P-Genus) FISH assay has a Plasmodium genus specific probe that detects all five species of Plasmodium known to cause the disease in humans. The P. falciparum (PF) FISH assay and P. vivax (PV) FISH assay detect and differentiate between P. falciparum and P. vivax respectively from other Plasmodium species. The FISH assays are more sensitive than Giemsa. The sensitivities of P-Genus, PF and PV FISH assays were found to be 98.2%, 94.5% and 98.3%, respectively compared to 89.9%, 83.3% and 87.9% for the detection of Plasmodium, P. falciparum and P. vivax by Giemsa staining respectively.
Conflict of interest statement
Figures

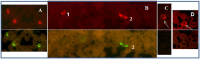


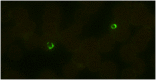
References
-
- WHO Geneva, Switzerland: WHO Press; 2. World Malaria Report 2014: 1–242,
-
- WHO Geneva, Switzerland: WHO Press; 2. World Malaria Report 2013: 1–255.
-
- CDC (2012) Malaria surveillance—United States, 2010. 1–17 p. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

