Novel BAC Mouse Model of Huntington's Disease with 225 CAG Repeats Exhibits an Early Widespread and Stable Degenerative Phenotype
- PMID: 26333255
- PMCID: PMC4657874
Novel BAC Mouse Model of Huntington's Disease with 225 CAG Repeats Exhibits an Early Widespread and Stable Degenerative Phenotype
Abstract
Background: Unusually large CAG repeat expansions (>60) in exon one of Huntingtin (HTT) are invariably associated with a juvenile-onset form of Huntington's disease (HD), characterized by a more extensive and rapidly progressing neuropathology than the more prevalent adult-onset form. However, existing mouse models of HD that express the full-length Htt gene with CAG repeat lengths associated with juvenile HD (ranging between ~75 to ~150 repeats in published models) exhibit selective neurodegenerative phenotypes more consistent with adult-onset HD. Objective: To determine if a very large CAG repeat (>200) in full-length Htt elicits neurodegenerative phenotypes consistent with juvenile HD.
Methods: Using a …bacterial artificial chromosome (BAC) system, we generated mice expressing full-length mouse Htt with ~225 CAG repeats under control of the mouse Htt promoter. Mice were characterized using behavioral, neuropathological, biochemical and brain imaging methods.
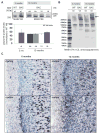
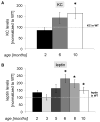
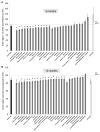
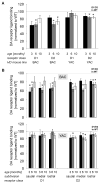
Results: BAC-225Q mice exhibit phenotypes consistent with a subset of features seen in juvenile-onset HD: very early motor behavior abnormalities, reduced body weight, widespread and progressive increase in Htt aggregates, gliosis, and neurodegeneration. Early striatal pathology was observed, including reactive gliosis and loss of dopamine receptors, prior to detectable volume loss. HD-related blood markers of impaired energy metabolism and systemic inflammation were also increased. Aside from an age-dependent progression of diffuse nuclear aggregates at 6 months of age to abundant neuropil aggregates at 12 months of age, other pathological and motor phenotypes showed little to no progression.
Conclusions: The HD phenotypes present in animals 3 to 12 months of age make the BAC-225Q mice a unique and stable model of full-length mutant Htt associated phenotypes, including body weight loss, behavioral impairment and HD-like neurodegenerative phenotypes characteristic of juvenile-onset HD and/or late-stage adult-onset HD.
Conflict of interest statement
The authors have no conflict of interest to report.
Figures




Similar articles
-
Transgenic mice expressing mutated full-length HD cDNA: a paradigm for locomotor changes and selective neuronal loss in Huntington's disease.Philos Trans R Soc Lond B Biol Sci. 1999 Jun 29;354(1386):1035-45. doi: 10.1098/rstb.1999.0456. Philos Trans R Soc Lond B Biol Sci. 1999. PMID: 10434303 Free PMC article.
-
Paradoxical delay in the onset of disease caused by super-long CAG repeat expansions in R6/2 mice.Neurobiol Dis. 2009 Mar;33(3):331-41. doi: 10.1016/j.nbd.2008.11.015. Epub 2008 Dec 11. Neurobiol Dis. 2009. PMID: 19130884
-
A novel BACHD transgenic rat exhibits characteristic neuropathological features of Huntington disease.J Neurosci. 2012 Oct 31;32(44):15426-38. doi: 10.1523/JNEUROSCI.1148-12.2012. J Neurosci. 2012. PMID: 23115180 Free PMC article.
-
Multiple clinical features of Huntington's disease correlate with mutant HTT gene CAG repeat lengths and neurodegeneration.J Neurol. 2019 Mar;266(3):551-564. doi: 10.1007/s00415-018-8940-6. Epub 2018 Jun 28. J Neurol. 2019. PMID: 29956026 Review.
-
Selective degeneration in YAC mouse models of Huntington disease.Brain Res Bull. 2007 Apr 30;72(2-3):124-31. doi: 10.1016/j.brainresbull.2006.10.018. Epub 2006 Nov 16. Brain Res Bull. 2007. PMID: 17352936 Review.
Cited by
-
A New Perspective on Huntington's Disease: How a Neurological Disorder Influences the Peripheral Tissues.Int J Mol Sci. 2022 May 29;23(11):6089. doi: 10.3390/ijms23116089. Int J Mol Sci. 2022. PMID: 35682773 Free PMC article. Review.
-
Neuroinflammation in Huntington's Disease: A Starring Role for Astrocyte and Microglia.Curr Neuropharmacol. 2022;20(6):1116-1143. doi: 10.2174/1570159X19666211201094608. Curr Neuropharmacol. 2022. PMID: 34852742 Free PMC article. Review.
-
Rodent Models of Huntington's Disease: An Overview.Biomedicines. 2023 Dec 16;11(12):3331. doi: 10.3390/biomedicines11123331. Biomedicines. 2023. PMID: 38137552 Free PMC article. Review.
-
Do Changes in Synaptic Autophagy Underlie the Cognitive Impairments in Huntington's Disease?J Huntingtons Dis. 2021;10(2):227-238. doi: 10.3233/JHD-200466. J Huntingtons Dis. 2021. PMID: 33780373 Free PMC article. Review.
-
Early detection of exon 1 huntingtin aggregation in zQ175 brains by molecular and histological approaches.Brain Commun. 2023 Jan 20;5(1):fcad010. doi: 10.1093/braincomms/fcad010. eCollection 2023. Brain Commun. 2023. PMID: 36756307 Free PMC article.
References
-
- Walker FO. Huntington's Disease. Seminars in neurology. 2007;27(2):143–50. - PubMed
-
- MacDonald ME, Ambrose CM, Duyao MP, Myers RH, Lin C, Srinidhi L, Barnes G, Taylor SA, James M, Groot M, MacFarlane H, Jenkins B. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. The Huntington's Disease Collaborative Research Group. Cell. 1993;72(6):971–83. - PubMed
-
- Douaud G, Gaura V, Ribeiro MJ, Lethimonnier F, Maroy R, Verny C, Krystkowiak P, Damier P, Bachoud-Levi AC, Hantraye P, Remy P. Distribution of grey matter atrophy in Huntington's disease patients: a combined ROI-based and voxel-based morphometric study. Neuroimage. 2006;32(4):1562–75. - PubMed
-
- Boutell JM, Thomas P, Neal JW, Weston VJ, Duce J, Harper PS, Jones AL. Aberrant interactions of transcriptional repressor proteins with the Huntington's disease gene product, huntingtin. Hum Mol Genet. 1999;8(9):1647–55. - PubMed
-
- Sassone J, Colciago C, Cislaghi G, Silani V, Ciammola A. Huntington's disease: the current state of research with peripheral tissues. Exp Neurol. 2009;219(2):385–97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 MH015330/MH/NIMH NIH HHS/United States
- R01 ES007062/ES/NIEHS NIH HHS/United States
- T32ES007322/ES/NIEHS NIH HHS/United States
- P30 HD015052/HD/NICHD NIH HHS/United States
- P30HD15052/HD/NICHD NIH HHS/United States
- T32 ES007028/ES/NIEHS NIH HHS/United States
- T32 ES007322/ES/NIEHS NIH HHS/United States
- R01 ES016931/ES/NIEHS NIH HHS/United States
- T32MH015330/MH/NIMH NIH HHS/United States
- T32ES007028/ES/NIEHS NIH HHS/United States
- R01ES007062/ES/NIEHS NIH HHS/United States
- P30 ES009089/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
