Efficacy of transfusion with granulocytes from G-CSF/dexamethasone-treated donors in neutropenic patients with infection
- PMID: 26333778
- PMCID: PMC4626256
- DOI: 10.1182/blood-2015-05-645986
Efficacy of transfusion with granulocytes from G-CSF/dexamethasone-treated donors in neutropenic patients with infection
Abstract
High-dose granulocyte transfusion therapy has been available for 20 years, yet its clinical efficacy has never been conclusively demonstrated. We report here the results of RING (Resolving Infection in Neutropenia with Granulocytes), a multicenter randomized controlled trial designed to address this question. Eligible subjects were those with neutropenia (absolute neutrophil count <500/μL) and proven/probable/presumed infection. Subjects were randomized to receive either (1) standard antimicrobial therapy or (2) standard antimicrobial therapy plus daily granulocyte transfusions from donors stimulated with granulocyte colony-stimulating factor (G-CSF) and dexamethasone. The primary end point was a composite of survival plus microbial response, at 42 days after randomization. Microbial response was determined by a blinded adjudication panel. Fifty-six subjects were randomized to the granulocyte arm and 58 to the control arm. Transfused subjects received a median of 5 transfusions. Mean transfusion dose was 54.9 × 10(9) granulocytes. Overall success rates were 42% and 43% for the granulocyte and control groups, respectively (P > .99), and 49% and 41%, respectively, for subjects who received their assigned treatments (P = .64). Success rates for granulocyte and control arms did not differ within any infection type. In a post hoc analysis, subjects who received an average dose per transfusion of ≥0.6 × 10(9) granulocytes per kilogram tended to have better outcomes than those receiving a lower dose. In conclusion, there was no overall effect of granulocyte transfusion on the primary outcome, but because enrollment was half that planned, power to detect a true beneficial effect was low. RING was registered at www.clinicaltrials.gov as #NCT00627393.
© 2015 by The American Society of Hematology.
Figures
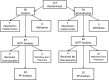



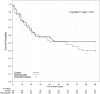
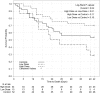
Comment in
-
Granulocyte transfusion: questions remain.Blood. 2015 Oct 29;126(18):2082-3. doi: 10.1182/blood-2015-09-669085. Blood. 2015. PMID: 26516218 Free PMC article.
References
-
- Grow WB, Moreb JS, Roque D, et al. Late onset of invasive aspergillus infection in bone marrow transplant patients at a university hospital. Bone Marrow Transplant. 2002;29(1):15–19. - PubMed
-
- Marr KA, Schlamm HT, Herbrecht R, et al. Combination antifungal therapy for invasive aspergillosis: a randomized trial. Ann Intern Med. 2015;162(2):81–89. - PubMed
-
- Wingard JR, Carter SL, Walsh TJ, et al. Blood and Marrow Transplant Clinical Trials Network. Randomized, double-blind trial of fluconazole versus voriconazole for prevention of invasive fungal infection after allogeneic hematopoietic cell transplantation. Blood. 2010;116(24):5111–5118. - PMC - PubMed
-
- Ullmann AJ, Lipton JH, Vesole DH, et al. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med. 2007;356(4):335–347. - PubMed
-
- Robenshtok E, Gafter-Gvili A, Goldberg E, et al. Antifungal prophylaxis in cancer patients after chemotherapy or hematopoietic stem-cell transplantation: systematic review and meta-analysis. J Clin Oncol. 2007;25(34):5471–5489. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- HL072321/HL/NHLBI NIH HHS/United States
- U01 HL072346/HL/NHLBI NIH HHS/United States
- HL072290/HL/NHLBI NIH HHS/United States
- U01 HL072305/HL/NHLBI NIH HHS/United States
- U01 HL072028/HL/NHLBI NIH HHS/United States
- HL072268/HL/NHLBI NIH HHS/United States
- UL1 TR000427/TR/NCATS NIH HHS/United States
- HL072196/HL/NHLBI NIH HHS/United States
- U01 HL072291/HL/NHLBI NIH HHS/United States
- HL072283/HL/NHLBI NIH HHS/United States
- HL072191/HL/NHLBI NIH HHS/United States
- HL072072/HL/NHLBI NIH HHS/United States
- U01 HL072290/HL/NHLBI NIH HHS/United States
- HL072346/HL/NHLBI NIH HHS/United States
- HL072305/HL/NHLBI NIH HHS/United States
- U01 HL072283/HL/NHLBI NIH HHS/United States
- U01 HL072072/HL/NHLBI NIH HHS/United States
- R01 HL072321/HL/NHLBI NIH HHS/United States
- HL072291/HL/NHLBI NIH HHS/United States
- U01 HL072191/HL/NHLBI NIH HHS/United States
- U01 HL072196/HL/NHLBI NIH HHS/United States
- HL072028/HL/NHLBI NIH HHS/United States
- U01 HL072268/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

