Thromboprophylaxis with apixaban and the risk of pulmonary embolism in patients undergoing knee replacement surgery
- PMID: 26333856
- PMCID: PMC4558285
- DOI: 10.3402/jchimp.v5.27889
Thromboprophylaxis with apixaban and the risk of pulmonary embolism in patients undergoing knee replacement surgery
Abstract
Background: Apixaban, a novel oral anticoagulant, is also used for deep vein thrombosis (DVT) prophylaxis. In this study, we sought to critically evaluate the differences in the rates of symptomatic DVT and bleeding, and analyze the rates of pulmonary embolism (PE) in subgroups of patients from ADVANCE I and II trials given their similar indication and design.
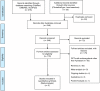
Methods: Studies were identified through electronic literature searches of MEDLINE, clinicaltrial.gov, SCOPUS, and EMBASE up to January 2014. Phase III RCTs involving use of apixaban and enoxaparin for thromboprophylaxis in patients undergoing total knee or hip replacement were included. Study-specific odds ratios were calculated and between-study heterogeneity was assessed using the I (2) statistics.
Results: In three studies involving 11,659 patients, the risk of symptomatic DVT (pooled OR 0.38, 95% CI 0.16-0.90, I (2)=0%, p=0.03) and bleeding (pooled OR 0.87, 95% CI 0.77-0.99, I (2)=0%, p=0.03) was less in apixaban group compared to the enoxaparin group. However, it was interesting to note that on subgroup analysis, the risk of PE was higher with apixaban when used for thromboprophylaxis in knee replacement surgery (pooled OR 2.58, 95% CI 1.10-6.04, I (2)=0%, p=0.03).
Conclusion: Apixaban was found to be associated with lower risk of symptomatic DVT and bleeding compared to enoxaparin when used for thromboprophylaxis in patients undergoing knee and hip replacement surgeries. However, it was associated with higher risk of PE in patients undergoing knee replacement.
Keywords: apixaban; arthroplasty; hip replacement; knee replacement; meta-analysis.
Figures
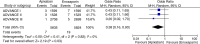
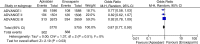
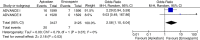
Similar articles
-
Apixaban versus enoxaparin for thromboprophylaxis after total hip or knee arthroplasty: a meta-analysis of randomized controlled trials.Chin Med J (Engl). 2012 Jul;125(13):2339-45. Chin Med J (Engl). 2012. PMID: 22882859
-
Direct factor Xa inhibitors (rivaroxaban and apixaban) versus enoxaparin for the prevention of venous thromboembolism after total knee replacement: A meta-analysis of 6 randomized clinical trials.Thromb Res. 2015 May;135(5):816-22. doi: 10.1016/j.thromres.2015.02.008. Epub 2015 Feb 12. Thromb Res. 2015. PMID: 25728496
-
Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE-2): a randomised double-blind trial.Lancet. 2010 Mar 6;375(9717):807-15. doi: 10.1016/S0140-6736(09)62125-5. Lancet. 2010. PMID: 20206776 Clinical Trial.
-
The role of apixaban for venous and arterial thromboembolic disease.Ann Pharmacother. 2011 Oct;45(10):1262-83. doi: 10.1345/aph.1Q119. Epub 2011 Sep 27. Ann Pharmacother. 2011. PMID: 21954450 Review.
-
Thromboprophylaxis With Apixaban in Patients Undergoing Major Orthopedic Surgery: Meta-Analysis and Trial-Sequential Analysis.Clin Med Insights Blood Disord. 2017 May 8;10:1179545X17704660. doi: 10.1177/1179545X17704660. eCollection 2017. Clin Med Insights Blood Disord. 2017. PMID: 28579855 Free PMC article. Review.
Cited by
-
Editor's notes.J Community Hosp Intern Med Perspect. 2015 Sep 1;5(4):29367. doi: 10.3402/jchimp.v5.29367. eCollection 2015. J Community Hosp Intern Med Perspect. 2015. PMID: 26333868 Free PMC article. No abstract available.
-
Comparison of Apixaban and Low Molecular Weight Heparin in Preventing Deep Venous Thrombosis after Total Knee Arthroplasty in Older Adults.Yonsei Med J. 2019 Jul;60(7):626-632. doi: 10.3349/ymj.2019.60.7.626. Yonsei Med J. 2019. PMID: 31250576 Free PMC article. Clinical Trial.
-
High-dose (3 g) topical tranexamic acid has higher potency in reducing blood loss after total knee arthroplasty compared with low dose (500 mg): a double-blind randomized controlled trial.Eur J Orthop Surg Traumatol. 2019 Dec;29(8):1729-1735. doi: 10.1007/s00590-019-02515-2. Epub 2019 Jul 29. Eur J Orthop Surg Traumatol. 2019. PMID: 31359178 Clinical Trial.
-
The role of new oral anticoagulants in orthopaedics: an update of recent evidence.Eur J Orthop Surg Traumatol. 2017 Jul;27(5):573-582. doi: 10.1007/s00590-017-1940-x. Epub 2017 Mar 17. Eur J Orthop Surg Traumatol. 2017. PMID: 28314985 Review.
References
-
- Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition) Chest. 2008;133(6 Suppl):381S–453S. - PubMed
-
- Levine MN, Hirsh J, Gent M, Turpie AG, Leclerc J, Powers PJ, et al. Prevention of deep vein thrombosis after elective hip surgery. A randomized trial comparing low molecular weight heparin with standard unfractionated heparin. Ann Intern Med. 1991;114(7):545–51. - PubMed
-
- Hull R, Raskob G, Pineo G, Rosenbloom D, Evans W, Mallory T, et al. A comparison of subcutaneous low-molecular-weight heparin with warfarin sodium for prophylaxis against deep-vein thrombosis after hip or knee implantation. N Engl J Med. 1993;329(19):1370–6. - PubMed
-
- Ansell J, Hirsh J, Poller L, Bussey H, Jacobson A, Hylek E. The pharmacology and management of the vitamin K antagonists: The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 Suppl):204S–33S. - PubMed
-
- Dahl OE, Pleil AM. Investment in prolonged thromboprophylaxis with dalteparin improves clinical outcomes after hip replacement. J Thromb Haemost. 2003;1(5):896–906. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
