Processing time of addition or withdrawal of single or combined balance-stabilizing haptic and visual information
- PMID: 26334013
- PMCID: PMC4686285
- DOI: 10.1152/jn.00618.2015
Processing time of addition or withdrawal of single or combined balance-stabilizing haptic and visual information
Abstract
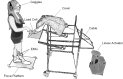
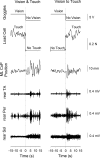
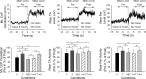
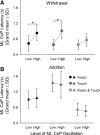
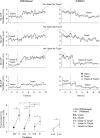
We investigated the integration time of haptic and visual input and their interaction during stance stabilization. Eleven subjects performed four tandem-stance conditions (60 trials each). Vision, touch, and both vision and touch were added and withdrawn. Furthermore, vision was replaced with touch and vice versa. Body sway, tibialis anterior, and peroneus longus activity were measured. Following addition or withdrawal of vision or touch, an integration time period elapsed before the earliest changes in sway were observed. Thereafter, sway varied exponentially to a new steady-state while reweighting occurred. Latencies of sway changes on sensory addition ranged from 0.6 to 1.5 s across subjects, consistently longer for touch than vision, and were regularly preceded by changes in muscle activity. Addition of vision and touch simultaneously shortened the latencies with respect to vision or touch separately, suggesting cooperation between sensory modalities. Latencies following withdrawal of vision or touch or both simultaneously were shorter than following addition. When vision was replaced with touch or vice versa, adding one modality did not interfere with the effect of withdrawal of the other, suggesting that integration of withdrawal and addition were performed in parallel. The time course of the reweighting process to reach the new steady-state was also shorter on withdrawal than addition. The effects of different sensory inputs on posture stabilization illustrate the operation of a time-consuming, possibly supraspinal process that integrates and fuses modalities for accurate balance control. This study also shows the facilitatory interaction of visual and haptic inputs in integration and reweighting of stance-stabilizing inputs.
Keywords: haptic; sensory integration; sensory reweighting; standing; vision.
Copyright © 2015 the American Physiological Society.
Figures



References
-
- Azañón E, Longo MR, Soto-Faraco S, Haggard P. The posterior parietal cortex remaps touch into external space. Curr Biol 20: 1304–1309, 2010. - PubMed
-
- Baudry S, Duchateau J. Independent modulation of corticospinal and group I afferents pathways during upright standing. Neuroscience 275: 162–169, 2014. - PubMed
-
- Baudry S, Penzer F, Duchateau J. Vision and proprioception do not influence the excitability of the corticomotoneuronal pathway during upright standing in young and elderly adults. Neuroscience 268: 247–254, 2014. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

