Impact of cercal air currents on singing motor pattern generation in the cricket (Gryllus bimaculatus DeGeer)
- PMID: 26334014
- PMCID: PMC4643095
- DOI: 10.1152/jn.00669.2015
Impact of cercal air currents on singing motor pattern generation in the cricket (Gryllus bimaculatus DeGeer)
Abstract
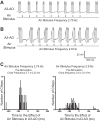
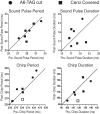
The cercal system of crickets detects low-frequency air currents produced by approaching predators and self-generated air currents during singing, which may provide sensory feedback to the singing motor network. We analyzed the effect of cercal stimulation on singing motor pattern generation to reveal the response of a singing interneuron to predator-like signals and to elucidate the possible role of self-generated air currents during singing. In fictive singing males, we recorded an interneuron of the singing network while applying air currents to the cerci; additionally, we analyzed the effect of abolishing the cercal system in freely singing males. In fictively singing crickets, the effect of short air stimuli is either to terminate prematurely or to lengthen the interchirp interval, depending on their phase in the chirp cycle. Within our stimulation paradigm, air stimuli of different velocities and durations always elicited an inhibitory postsynaptic potential in the singing interneuron. Current injection in the singing interneuron elicited singing motor activity, even during the air current-evoked inhibitory input from the cercal pathway. The disruptive effects of air stimuli on the fictive singing pattern and the inhibitory response of the singing interneuron point toward the cercal system being involved in initiating avoidance responses in singing crickets, according to the established role of cerci in a predator escape pathway. After abolishing the activity of the cercal system, the timing of natural singing activity was not significantly altered. Our study provides no evidence that self-generated cercal sensory activity has a feedback function for singing motor pattern generation.
Keywords: air stimulus; cercal sensory system; escape response; singing central pattern generator interneuron.
Copyright © 2015 the American Physiological Society.
Figures





References
-
- Andersson O, Forssberg H, Grillner S, Wallén P. Peripheral feedback mechanisms acting on the central pattern generators for locomotion in fish and cat. Can J Physiol Pharmacol 59: 713–726, 1981. - PubMed
-
- ASAB Ethics Committee. Guidelines for the treatment of animals in behavioural research and teaching. Anim Behav 71: 245–253, 2006. - PubMed
-
- Baba Y, Shimozawa T. Diversity of motor responses initiated by a wind stimulus in the freely moving cricket, Gryllus bimaculatus. Zoolog Sci 14: 587–594, 1997.
-
- Bässler U. Afferent control of walking movements in the stick insect Cuniculina impigra. J Comp Physiol A 158: 351–362, 1986.
-
- Bellman KL, Krasne FB. Adaptive complexity of interactions between feeding and escape in crayfish. Science 221: 779–781, 1983. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

