Molecular dissection of HBV evasion from restriction factor tetherin: A new perspective for antiviral cell therapy
- PMID: 26334101
- PMCID: PMC4673130
- DOI: 10.18632/oncotarget.4808
Molecular dissection of HBV evasion from restriction factor tetherin: A new perspective for antiviral cell therapy
Abstract
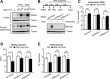
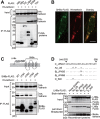
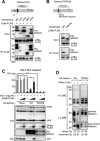
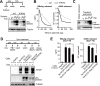
Viruses have evolved various strategies to escape from the innate cellular mechanisms inhibiting viral replication and spread. Extensive evidence has highlighted the ineffectiveness of interferon (IFN) therapy against chronic hepatitis B virus (HBV) infection, implying the existence of mechanisms by which HBV evades IFN-induced antiviral responses. In our current study, we demonstrate that HBV surface protein (HBs) plays a crucial role in counteracting the IFN-induced antiviral response mediated by tetherin (also known as BST-2). The type I IFN treatment of HBV-producing cells marginally but significantly inhibited the release of HBsAg and viral DNA, but this release was recovered by the knockdown of tetherin. HBs can interact with tetherin via its fourth transmembrane domain thereby inhibiting its dimerization and antiviral activity. The expression of a tetherin mutant devoid of the HBs-binding domain promoted a prominent restriction of HBV particle production that eventually resulted in the alleviation of caspase-1-mediated cytotoxicity and interleukin-1β secretion in induced pluripotent stem cell (iPSC)-derived hepatocytes. Our current results thus reveal a previously undescribed molecular link between HBV and tetherin during the course of an IFN-induced antiviral response. In addition, strategies to augment the antiviral activity of tetherin by impeding tetherin-HBs interactions may be viable as a therapeutic intervention against HBV.
Keywords: Immune response; Immunity; Immunology and Microbiology Section; hepatic injury; pyroptosis.
Conflict of interest statement
No, there is no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

