Solution Structure of a Nonribosomal Peptide Synthetase Carrier Protein Loaded with Its Substrate Reveals Transient, Well-Defined Contacts
- PMID: 26334259
- PMCID: PMC4689197
- DOI: 10.1021/jacs.5b07772
Solution Structure of a Nonribosomal Peptide Synthetase Carrier Protein Loaded with Its Substrate Reveals Transient, Well-Defined Contacts
Abstract
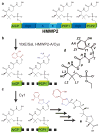
Nonribosomal peptide synthetases (NRPSs) are microbial enzymes that produce a wealth of important natural products by condensing substrates in an assembly line manner. The proper sequence of substrates is obtained by tethering them to phosphopantetheinyl arms of holo carrier proteins (CPs) via a thioester bond. CPs in holo and substrate-loaded forms visit NRPS catalytic domains in a series of transient interactions. A lack of structural information on substrate-loaded carrier proteins has hindered our understanding of NRPS synthesis. Here, we present the first structure of an NRPS aryl carrier protein loaded with its substrate via a native thioester bond, together with the structure of its holo form. We also present the first quantification of NRPS CP backbone dynamics. Our results indicate that prosthetic moieties in both holo and loaded forms are in contact with the protein core, but they also sample states in which they are disordered and extend in solution. We observe that substrate loading induces a large conformational change in the phosphopantetheinyl arm, thereby modulating surfaces accessible for binding to other domains. Our results are discussed in the context of NRPS domain interactions.
Figures
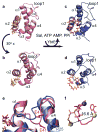


Similar articles
-
Molecular impact of covalent modifications on nonribosomal peptide synthetase carrier protein communication.J Biol Chem. 2017 Jun 16;292(24):10002-10013. doi: 10.1074/jbc.M116.766220. Epub 2017 Apr 28. J Biol Chem. 2017. PMID: 28455448 Free PMC article.
-
Purification, priming, and catalytic acylation of carrier protein domains in the polyketide synthase and nonribosomal peptidyl synthetase modules of the HMWP1 subunit of yersiniabactin synthetase.Proc Natl Acad Sci U S A. 2001 Jan 2;98(1):99-104. doi: 10.1073/pnas.98.1.99. Proc Natl Acad Sci U S A. 2001. PMID: 11134531 Free PMC article.
-
Structural insight into the necessary conformational changes of modular nonribosomal peptide synthetases.Curr Opin Chem Biol. 2016 Dec;35:89-96. doi: 10.1016/j.cbpa.2016.09.005. Epub 2016 Sep 25. Curr Opin Chem Biol. 2016. PMID: 27676239 Free PMC article. Review.
-
Yersiniabactin synthetase: probing the recognition of carrier protein domains by the catalytic heterocyclization domains, Cy1 and Cy2, in the chain-initiating HWMP2 subunit.Biochemistry. 2001 May 1;40(17):5313-21. doi: 10.1021/bi002905v. Biochemistry. 2001. PMID: 11318656
-
New Structural Data Reveal the Motion of Carrier Proteins in Nonribosomal Peptide Synthesis.Angew Chem Int Ed Engl. 2016 Aug 16;55(34):9834-40. doi: 10.1002/anie.201602614. Epub 2016 Jul 20. Angew Chem Int Ed Engl. 2016. PMID: 27435901 Free PMC article. Review.
Cited by
-
Finding Druggable Sites in Proteins Using TACTICS.J Chem Inf Model. 2021 Jun 28;61(6):2897-2910. doi: 10.1021/acs.jcim.1c00204. Epub 2021 Jun 7. J Chem Inf Model. 2021. PMID: 34096704 Free PMC article.
-
Using delayed decoupling to attenuate residual signals in editing filters.Magn Reson (Gott). 2021;2(1):475-487. doi: 10.5194/mr-2-475-2021. Epub 2021 Jun 21. Magn Reson (Gott). 2021. PMID: 34661195 Free PMC article.
-
Cooperation between a T Domain and a Minimal C-Terminal Docking Domain to Enable Specific Assembly in a Multiprotein NRPS.Angew Chem Int Ed Engl. 2021 Jun 14;60(25):14171-14178. doi: 10.1002/anie.202103498. Epub 2021 May 14. Angew Chem Int Ed Engl. 2021. PMID: 33876501 Free PMC article.
-
Insights into Thiotemplated Pyrrole Biosynthesis Gained from the Crystal Structure of Flavin-Dependent Oxidase in Complex with Carrier Protein.Biochemistry. 2019 Feb 19;58(7):918-929. doi: 10.1021/acs.biochem.8b01177. Epub 2019 Jan 23. Biochemistry. 2019. PMID: 30620182 Free PMC article.
-
FRET monitoring of a nonribosomal peptide synthetase.Nat Chem Biol. 2017 Sep;13(9):1009-1015. doi: 10.1038/nchembio.2435. Epub 2017 Jul 24. Nat Chem Biol. 2017. PMID: 28759017
References
-
- Khosla C. Chem Rev. 1997;97:2577. - PubMed
-
- Jenni S, Leibundgut M, Maier T, Ban N. Science. 2006;311:1263. - PubMed
-
- Jenni S, Leibundgut M, Boehringer D, Frick C, Mikolasek B, Ban N. Science. 2007;316:254. - PubMed
-
- Leibundgut M, Jenni S, Frick C, Ban N. Science. 2007;316:288. - PubMed
-
- Khosla C. J Org Chem. 2009;74:6416. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

