Distinct but Spatially Overlapping Intestinal Niches for Vancomycin-Resistant Enterococcus faecium and Carbapenem-Resistant Klebsiella pneumoniae
- PMID: 26334306
- PMCID: PMC4559429
- DOI: 10.1371/journal.ppat.1005132
Distinct but Spatially Overlapping Intestinal Niches for Vancomycin-Resistant Enterococcus faecium and Carbapenem-Resistant Klebsiella pneumoniae
Abstract
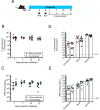
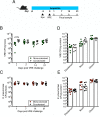
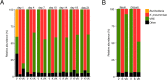
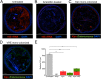
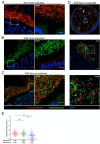
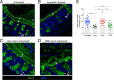
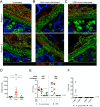
Antibiotic resistance among enterococci and γ-proteobacteria is an increasing problem in healthcare settings. Dense colonization of the gut by antibiotic-resistant bacteria facilitates their spread between patients and also leads to bloodstream and other systemic infections. Antibiotic-mediated destruction of the intestinal microbiota and consequent loss of colonization resistance are critical factors leading to persistence and spread of antibiotic-resistant bacteria. The mechanisms underlying microbiota-mediated colonization resistance remain incompletely defined and are likely distinct for different antibiotic-resistant bacterial species. It is unclear whether enterococci or γ-proteobacteria, upon expanding to high density in the gut, confer colonization resistance against competing bacterial species. Herein, we demonstrate that dense intestinal colonization with vancomycin-resistant Enterococcus faecium (VRE) does not reduce in vivo growth of carbapenem-resistant Klebsiella pneumoniae. Reciprocally, K. pneumoniae does not impair intestinal colonization by VRE. In contrast, transplantation of a diverse fecal microbiota eliminates both VRE and K. pneumoniae from the gut. Fluorescence in situ hybridization demonstrates that VRE and K. pneumoniae localize to the same regions in the colon but differ with respect to stimulation and invasion of the colonic mucus layer. While VRE and K. pneumoniae occupy the same three-dimensional space within the gut lumen, their independent growth and persistence in the gut suggests that they reside in distinct niches that satisfy their specific in vivo metabolic needs.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Safdar N, Maki DG. The commonality of risk factors for nosocomial colonization and infection with antimicrobial-resistant staphylococcus aureus, enterococcus, gram-negative bacilli, clostridium difficile, and candida. Ann Intern Med 2002, June 4;136(11):834–44. - PubMed
-
- Donskey CJ. The role of the intestinal tract as a reservoir and source for transmission of nosocomial pathogens. Clin Infect Dis 2004, July 15;39(2):219–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

