Pharmacokinetics and Bioavailability of a Fixed-Dose Combination of Ibuprofen and Paracetamol after Intravenous and Oral Administration
- PMID: 26334726
- PMCID: PMC4579261
- DOI: 10.1007/s40261-015-0320-8
Pharmacokinetics and Bioavailability of a Fixed-Dose Combination of Ibuprofen and Paracetamol after Intravenous and Oral Administration
Abstract
Background and objectives: Previously published studies have suggested the lack of a pharmacokinetic interaction between ibuprofen and paracetamol when they are delivered as a fixed-dose oral combination. The aim of this study was to determine the pharmacokinetic profile and safety of a fixed-dose intravenous (IV) combination, containing 3 mg/mL ibuprofen and 10 mg/mL paracetamol, in comparison with its individual components. The study also assessed the relative bioavailability of the same doses of the active ingredients when they were administered as an oral formulation.
Methods: A single-dose, open-label, randomized, five-period cross-over sequence pharmacokinetic study was undertaken in 30 healthy volunteers. Serial plasma samples were assayed for both paracetamol and ibuprofen concentrations, using validated liquid chromatography-tandem mass spectrometry methods. Pharmacokinetic parameters were computed using standard non-compartmental analyses. Adverse events were also assessed. The ratios of the maximum measured plasma concentration (C max), the area under the plasma concentration-time curve (AUC) from time zero to the time of the last measurable plasma concentration (AUCt ) and AUC from time zero to infinity (AUC∞) were analysed for bioequivalence as determined by 90% confidence intervals.
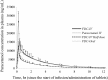
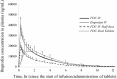
Results: The pharmacokinetic parameters of ibuprofen and paracetamol were very similar for the combination and monotherapy IV preparations; the ratios of the C max, AUC t and AUC∞ values fell within the 80-125% acceptable bioequivalence range. Precise dose proportionality for both compounds was also determined for the half dose of the IV formulation in comparison with the full dose. The relative bioavailability of paracetamol (93.78%) and ibuprofen (96.45%) confirmed the pharmacokinetic equivalence of the oral and IV formulations of the fixed-dose combination.
Conclusion: Concomitant administration of 3 mg/mL ibuprofen and 10 mg/mL paracetamol in a fixed-dose IV combination does not alter the pharmacokinetic profiles of either drug. The IV and oral dose forms of such a combination are pharmacokinetically equivalent.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

