Differential Subcellular Localization of Leishmania Alba-Domain Proteins throughout the Parasite Development
- PMID: 26334886
- PMCID: PMC4559404
- DOI: 10.1371/journal.pone.0137243
Differential Subcellular Localization of Leishmania Alba-Domain Proteins throughout the Parasite Development
Abstract
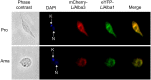
Alba-domain proteins are RNA-binding proteins found in archaea and eukaryotes and recently studied in protozoan parasites where they play a role in the regulation of virulence factors and stage-specific proteins. This work describes in silico structural characterization, cellular localization and biochemical analyses of Alba-domain proteins in Leishmania infantum. We show that in contrast to other protozoa, Leishmania have two Alba-domain proteins, LiAlba1 and LiAlba3, representative of the Rpp20- and the Rpp25-like eukaryotic subfamilies, respectively, which share several sequence and structural similarities but also important differences with orthologs in other protozoa, especially in sequences targeted for post-translational modifications. LiAlba1 and LiAlba3 proteins form a complex interacting with other RNA-binding proteins, ribosomal subunits, and translation factors as supported by co-immunoprecipitation and sucrose gradient sedimentation analysis. A higher co-sedimentation of Alba proteins with ribosomal subunits was seen upon conditions of decreased translation, suggesting a role of these proteins in translational repression. The Leishmania Alba-domain proteins display differential cellular localization throughout the parasite development. In the insect promastigote stage, Alba proteins co-localize predominantly to the cytoplasm but they translocate to the nucleolus and the flagellum upon amastigote differentiation in the mammalian host and are found back to the cytoplasm once amastigote differentiation is completed. Heat-shock, a major signal of amastigote differentiation, triggers Alba translocation to the nucleolus and the flagellum. Purification of the Leishmania flagellum confirmed LiAlba3 enrichment in this organelle during amastigote differentiation. Moreover, partial characterization of the Leishmania flagellum proteome of promastigotes and differentiating amastigotes revealed the presence of other RNA-binding proteins, as well as differences in the flagellum composition between these two parasite lifestages. Shuttling of Alba-domain proteins between the cytoplasm and the nucleolus or the flagellum throughout the parasite life cycle suggests that these RNA-binding proteins participate in several distinct regulatory pathways controlling developmental gene expression in Leishmania.
Conflict of interest statement
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

