Bentamapimod (JNK Inhibitor AS602801) Induces Regression of Endometriotic Lesions in Animal Models
- PMID: 26335175
- PMCID: PMC5933194
- DOI: 10.1177/1933719115600553
Bentamapimod (JNK Inhibitor AS602801) Induces Regression of Endometriotic Lesions in Animal Models
Abstract
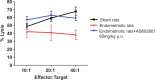
Endometriosis is an estrogen (ER)-dependent gynecological disease caused by the growth of endometrial tissue at extrauterine sites. Current endocrine therapies address the estrogenic aspect of disease and offer some relief from pain but are associated with significant side effects. Immune dysfunction is also widely believed to be an underlying contributor to the pathogenesis of this disease. This study evaluated an inhibitor of c-Jun N-terminal kinase, bentamapimod (AS602801), which interrupts immune pathways, in 2 rodent endometriosis models. Treatment of nude mice bearing xenografts biopsied from women with endometriosis (BWE) with 30 mg/kg AS602801 caused 29% regression of lesion. Medroxyprogesterone acetate (MPA) or progesterone (PR) alone did not cause regression of BWE lesions, but combining 10 mg/kg AS602801 with MPA caused 38% lesion regression. In human endometrial organ cultures (from healthy women), treatment with AS602801 or MPA reduced matrix metalloproteinase-3 (MMP-3) release into culture medium. In organ cultures established with BWE, PR or MPA failed to inhibit MMP-3 secretion, whereas AS602801 alone or MPA + AS602801 suppressed MMP-3 production. In an autologous rat endometriosis model, AS602801 caused 48% regression of lesions compared to GnRH antagonist Antide (84%). AS602801 reduced inflammatory cytokines in endometriotic lesions, while levels of cytokines in ipsilateral horns were unaffected. Furthermore, AS602801 enhanced natural killer cell activity, without apparent negative effects on uterus. These results indicate that bentamapimod induced regression of endometriotic lesions in endometriosis rodent animal models without suppressing ER action. c-Jun N-terminal kinase inhibition mediated a comprehensive reduction in cytokine secretion and moreover was able to overcome PR resistance.
Keywords: JNK inhibitor; MMP; MPA.; bentamapimod; cytokine; endometriosis; progesterone.
© The Author(s) 2015.
Conflict of interest statement
Figures






Similar articles
-
c-Jun NH2-terminal kinase inhibitor bentamapimod reduces induced endometriosis in baboons: an assessor-blind placebo-controlled randomized study.Fertil Steril. 2016 Mar;105(3):815-824.e5. doi: 10.1016/j.fertnstert.2015.11.022. Epub 2015 Dec 2. Fertil Steril. 2016. PMID: 26654972
-
The novel JNK inhibitor AS602801 inhibits cancer stem cells in vitro and in vivo.Oncotarget. 2016 May 10;7(19):27021-32. doi: 10.18632/oncotarget.8395. Oncotarget. 2016. PMID: 27027242 Free PMC article.
-
Reduction of sporadic and neurofibromatosis type 2-associated vestibular schwannoma growth in vitro and in vivo after treatment with the c-Jun N-terminal kinase inhibitor AS602801.J Neurosurg. 2022 Sep 9;138(4):962-971. doi: 10.3171/2022.7.JNS22934. Print 2023 Apr 1. J Neurosurg. 2022. PMID: 36087315 Free PMC article.
-
Kinase signalling pathways in endometriosis: potential targets for non-hormonal therapeutics.Hum Reprod Update. 2016 Apr;22(3):382-403. doi: 10.1093/humupd/dmv060. Epub 2016 Jan 5. Hum Reprod Update. 2016. PMID: 26740585 Review.
-
Animal models for anti-angiogenic therapy in endometriosis.J Reprod Immunol. 2013 Mar;97(1):85-94. doi: 10.1016/j.jri.2012.10.012. J Reprod Immunol. 2013. PMID: 23432875 Review.
Cited by
-
Regulation of NLRP3 Inflammasome by Phosphorylation.Front Immunol. 2018 Oct 8;9:2305. doi: 10.3389/fimmu.2018.02305. eCollection 2018. Front Immunol. 2018. PMID: 30349539 Free PMC article. Review.
-
WERF Endometriosis Phenome and Biobanking Harmonisation Project for Experimental Models in Endometriosis Research (EPHect-EM-Heterologous): heterologous rodent models.Mol Hum Reprod. 2025 Jul 3;31(3):gaaf022. doi: 10.1093/molehr/gaaf022. Mol Hum Reprod. 2025. PMID: 40628402 Free PMC article. Review.
-
Targeting Folate Metabolism Is Selectively Cytotoxic to Glioma Stem Cells and Effectively Cooperates with Differentiation Therapy to Eliminate Tumor-Initiating Cells in Glioma Xenografts.Int J Mol Sci. 2021 Oct 27;22(21):11633. doi: 10.3390/ijms222111633. Int J Mol Sci. 2021. PMID: 34769063 Free PMC article.
-
Endometriosis: An Immunologist's Perspective.Int J Mol Sci. 2025 May 28;26(11):5193. doi: 10.3390/ijms26115193. Int J Mol Sci. 2025. PMID: 40508002 Free PMC article. Review.
-
Neurotrophins and Their Receptors, Novel Therapeutic Targets for Pelvic Pain in Endometriosis, Are Coordinately Regulated by IL-1β via the JNK Signaling Pathway.Am J Pathol. 2023 Aug;193(8):1046-1058. doi: 10.1016/j.ajpath.2023.04.007. Epub 2023 May 8. Am J Pathol. 2023. PMID: 37164275 Free PMC article.
References
-
- Lebovic DI, Mueller MD, Taylor RN. Immunobiology of endometriosis. Fertil Steril. 2001;75(1):1–10. - PubMed
-
- Senturk LM, Arici A. Immunology of endometriosis. J Reprod Immunol. 1999;43(1):67–83. - PubMed
-
- Bulun SE, Cheng YH, Yin P, et al. Progesterone resistance in endometriosis: link to failure to metabolize estradiol. Mol Cell Endocrinol. 2006;248(1-2):94–103. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

