Circadian Rhythms in Cyanobacteria
- PMID: 26335718
- PMCID: PMC4557074
- DOI: 10.1128/MMBR.00036-15
Circadian Rhythms in Cyanobacteria
Abstract
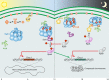
Life on earth is subject to daily and predictable fluctuations in light intensity, temperature, and humidity created by rotation of the earth. Circadian rhythms, generated by a circadian clock, control temporal programs of cellular physiology to facilitate adaptation to daily environmental changes. Circadian rhythms are nearly ubiquitous and are found in both prokaryotic and eukaryotic organisms. Here we introduce the molecular mechanism of the circadian clock in the model cyanobacterium Synechococcus elongatus PCC 7942. We review the current understanding of the cyanobacterial clock, emphasizing recent work that has generated a more comprehensive understanding of how the circadian oscillator becomes synchronized with the external environment and how information from the oscillator is transmitted to generate rhythms of biological activity. These results have changed how we think about the clock, shifting away from a linear model to one in which the clock is viewed as an interactive network of multifunctional components that are integrated into the context of the cell in order to pace and reset the oscillator. We conclude with a discussion of how this basic timekeeping mechanism differs in other cyanobacterial species and how information gleaned from work in cyanobacteria can be translated to understanding rhythmic phenomena in other prokaryotic systems.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures




References
-
- Mitsui A, Kumazawa S, Takahashi A, Ikemoto H, Cao S, Arai T. 1986. Strategy by which nitrogen-fixing unicellular cyanobacteria grow photoautotrophically. Nature 323:720–722. doi:10.1038/323720a0. - DOI
-
- Stal LJ, Krumbein WE. 1987. Temporal separation of nitrogen fixation and photosynthesis in the filamentous, non-heterocystous cyanobacterium Oscillatoria sp. Arch Microbiol 149:76–80. doi:10.1007/BF00423140. - DOI

