Alterations in the rostral ventromedial medulla after the selective ablation of μ-opioid receptor expressing neurons
- PMID: 26335909
- PMCID: PMC4829402
- DOI: 10.1097/j.pain.0000000000000344
Alterations in the rostral ventromedial medulla after the selective ablation of μ-opioid receptor expressing neurons
Abstract
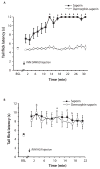
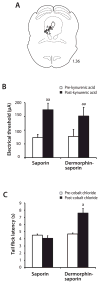
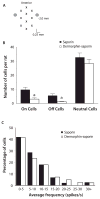
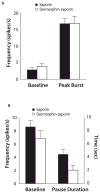
The rostral ventromedial medulla (RVM) exerts both inhibitory and excitatory controls over nociceptive neurons in the spinal cord and medullary dorsal horn. Selective ablation of mu-opioid receptor (MOR)-expressing neurons in the RVM using saporin conjugated to the MOR agonist dermorphin-saporin (derm-sap) attenuates stress and injury-induced behavioral hypersensitivity, yet the effect of RVM derm-sap on the functional integrity of the descending inhibitory system and the properties of RVM neurons remain unknown. Three classes of RVM neurons (on-cells, off-cells, and neutral cells) have been described with distinct responses to noxious stimuli and MOR agonists. Using single unit recording in lightly anesthetized rats, RVM neurons were characterized after microinjections of derm-sap or saporin. Derm-sap treatment resulted in a reduction in on-cells and off-cells when compared to saporin controls (P < 0.05). The number of neutral cells remained unchanged. After derm-sap treatment, RVM microinjections of the glutamate receptor agonist homocysteic acid increased tail-flick latencies, whereas the MOR agonist DAMGO had no effect. Furthermore, electrical stimulation of the periaqueductal gray produced analgesia in both derm-sap and saporin controls with similar thresholds. Microinjection of kynurenic acid, a glutamate receptor antagonist, into the RVM disrupted periaqueductal gray stimulation-produced analgesia in both saporin-treated and derm-sap-treated rats. These results indicate that MOR-expressing neurons in the RVM are not required for analgesia produced by either direct or indirect activation of neurons in the RVM.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
Similar articles
-
Inhibition of neuropathic pain by selective ablation of brainstem medullary cells expressing the mu-opioid receptor.J Neurosci. 2001 Jul 15;21(14):5281-8. doi: 10.1523/JNEUROSCI.21-14-05281.2001. J Neurosci. 2001. PMID: 11438603 Free PMC article.
-
Neuropathic pain is maintained by brainstem neurons co-expressing opioid and cholecystokinin receptors.Brain. 2009 Mar;132(Pt 3):778-87. doi: 10.1093/brain/awn330. Epub 2008 Dec 2. Brain. 2009. PMID: 19050032 Free PMC article.
-
Evaluation of side effects through selective ablation of the mu opioid receptor expressing descending nociceptive facilitatory neurons in the rostral ventromedial medulla with dermorphin-saporin.Neurotoxicology. 2009 Nov;30(6):1096-106. doi: 10.1016/j.neuro.2009.06.004. Epub 2009 Jun 24. Neurotoxicology. 2009. PMID: 19559047
-
Cell type-specific dissection of sensory pathways involved in descending modulation.Trends Neurosci. 2023 Jul;46(7):539-550. doi: 10.1016/j.tins.2023.04.002. Epub 2023 May 9. Trends Neurosci. 2023. PMID: 37164868 Free PMC article. Review.
-
Neuronal and glial factors contributing to sex differences in opioid modulation of pain.Neuropsychopharmacology. 2019 Jan;44(1):155-165. doi: 10.1038/s41386-018-0127-4. Epub 2018 Jun 23. Neuropsychopharmacology. 2019. PMID: 29973654 Free PMC article. Review.
Cited by
-
Cell-type specific molecular architecture for mu opioid receptor function in pain and addiction circuits.Neuropharmacology. 2023 Nov 1;238:109597. doi: 10.1016/j.neuropharm.2023.109597. Epub 2023 Jun 2. Neuropharmacology. 2023. PMID: 37271281 Free PMC article. Review.
-
Short-duration physical activity prevents the development of activity-induced hyperalgesia through opioid and serotoninergic mechanisms.Pain. 2017 Sep;158(9):1697-1710. doi: 10.1097/j.pain.0000000000000967. Pain. 2017. PMID: 28621702 Free PMC article.
-
Bulbospinal nociceptive ON and OFF cells related neural circuits and transmitters.Front Pharmacol. 2023 Apr 20;14:1159753. doi: 10.3389/fphar.2023.1159753. eCollection 2023. Front Pharmacol. 2023. PMID: 37153792 Free PMC article. Review.
-
G protein-coupled estrogen receptor in the rostral ventromedial medulla contributes to the chronification of postoperative pain.CNS Neurosci Ther. 2021 Nov;27(11):1313-1326. doi: 10.1111/cns.13704. Epub 2021 Jul 13. CNS Neurosci Ther. 2021. PMID: 34255932 Free PMC article.
-
Molecular identification of bulbospinal ON neurons by GPER, which drives pain and morphine tolerance.J Clin Invest. 2023 Jan 3;133(1):e154588. doi: 10.1172/JCI154588. J Clin Invest. 2023. PMID: 36346677 Free PMC article.
References
-
- Bederson JB, Fields HL, Barbaro NM. Hyperalgesia during naloxone-precipitated withdrawal from morphine is associated with increased on-cell activity in the rostral ventromedial medulla. Somatosens Mot Res. 1990;7:185–203. - PubMed
-
- Bee LA, Dickenson AH. Descending facilitation from the brainstem determines behavioural and neuronal hypersensitivity following nerve injury and efficacy of pregabalin. PAIN. 2008;140:209–23. - PubMed
-
- Behbehani MM, Fields HL. Evidence that an excitatory connection between the periaqueductal gray and nucleus raphe magnus mediates stimulation produced analgesia. Brain Res. 1979;170:85–93. - PubMed
-
- Beitz A. Relationship of glutamate and aspartate to the periaqueductal gray-raphe magnus projection: analysis using immunocytochemistry and microdialysis. J Histochem Cytochem. 1990;38:1755–65. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

