The Italian external quality assessment for RAS testing in colorectal carcinoma identifies methods-related inter-laboratory differences
- PMID: 26335936
- PMCID: PMC4557483
- DOI: 10.1186/s12967-015-0655-1
The Italian external quality assessment for RAS testing in colorectal carcinoma identifies methods-related inter-laboratory differences
Abstract
Background: In 2014 the European Medicines Agency included exon 2, 3 and 4 KRAS and NRAS testing for the selection of metastatic colorectal cancer (mCRC) patients eligible for the therapy with anti-EGFR monoclonal antibodies. The Italian Association of Medical Oncology (AIOM) and the Italian Society of Pathology and Cytology (SIAPEC) organized an external quality assessment (EQA) scheme for CRC to evaluate inter-laboratory consistency and to ensure standardization of the results in the transition from KRAS to all-RAS testing.
Methods: Ten formalin fixed paraffin embedded specimens including KRAS/NRAS (exons 2, 3, 4) and BRAF (codon 600) mutations were validated by three referral laboratories and sent to 88 participant centers. Molecular pathology sample reports were also requested to each laboratory. A board of assessors from AIOM and SIAPEC evaluated the results according to a predefined scoring system. The scheme was composed of two rounds.
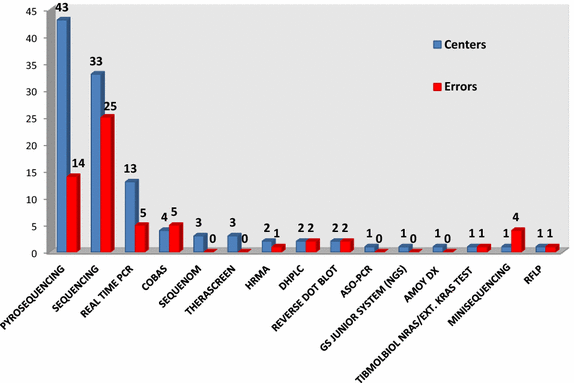
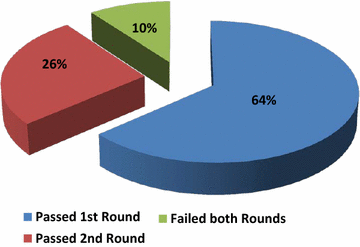
Results: In the first round 36% of the 88 participants failed, with 23 centers having at least one false positive or false negative while 9 centers did not meet the deadline. The genotyping error rate was higher when Sanger sequencing was employed for testing as compared with pyrosequencing (3 vs 1.3%; p = 0.01; Pearson Chi Square test). In the second round, the laboratories improved their performance, with 23/32 laboratories passing the round. Overall, 79/88 participants passed the RAS EQA scheme. Standardized Human Genome Variation Society nomenclature was incorrectly used to describe the mutations identified and relevant variations were noticed in the genotype specification.
Conclusion: The results of the Italian RAS EQA scheme indicate that the mutational analyses are performed with good quality in many Italian centers, although significant differences in the methods used were highlighted. The relatively high number of centers failing the first round underlines the fundamental role in continued education covered by EQA schemes.
Figures
Similar articles
-
Results of the First Italian External Quality Assurance Scheme for somatic EGFR mutation testing in non-small-cell lung cancer.J Thorac Oncol. 2013 Jun;8(6):773-8. doi: 10.1097/JTO.0b013e31828c2b08. J Thorac Oncol. 2013. PMID: 23575414
-
External quality assessment unravels interlaboratory differences in quality of RAS testing for anti-EGFR therapy in colorectal cancer.Oncologist. 2015 Mar;20(3):257-62. doi: 10.1634/theoncologist.2014-0382. Epub 2015 Feb 5. Oncologist. 2015. PMID: 25657200 Free PMC article.
-
KRAS mutations testing in colorectal carcinoma patients in Italy: from guidelines to external quality assessment.PLoS One. 2011;6(12):e29146. doi: 10.1371/journal.pone.0029146. Epub 2011 Dec 21. PLoS One. 2011. PMID: 22216189 Free PMC article.
-
Clinical implementation of KRAS testing in metastatic colorectal carcinoma: the pathologist's perspective.Arch Pathol Lab Med. 2012 Oct;136(10):1298-307. doi: 10.5858/arpa.2011-0478-RA. Epub 2012 Jan 24. Arch Pathol Lab Med. 2012. PMID: 22272560 Review.
-
RAS testing in metastatic colorectal cancer: advances in Europe.Virchows Arch. 2016 Apr;468(4):383-96. doi: 10.1007/s00428-015-1876-7. Epub 2015 Nov 16. Virchows Arch. 2016. PMID: 26573425 Free PMC article. Review.
Cited by
-
NGS for (Hemato-) Oncology in Belgium: Evaluation of Laboratory Performance and Feasibility of a National External Quality Assessment Program.Cancers (Basel). 2020 Oct 29;12(11):3180. doi: 10.3390/cancers12113180. Cancers (Basel). 2020. PMID: 33138022 Free PMC article.
-
Causes behind error rates for predictive biomarker testing: the utility of sending post-EQA surveys.Virchows Arch. 2021 May;478(5):995-1006. doi: 10.1007/s00428-020-02966-7. Epub 2020 Nov 23. Virchows Arch. 2021. PMID: 33225398 Free PMC article.
-
Prognostic value of carbohydrate antigen125 and carcino embryonic antigen expression in patients with colorectal carcinoma and its guiding significance for chemotherapy.Medicine (Baltimore). 2020 Apr;99(14):e19420. doi: 10.1097/MD.0000000000019420. Medicine (Baltimore). 2020. PMID: 32243362 Free PMC article.
-
RAS testing for colorectal cancer patients is reliable in European laboratories that pass external quality assessment.Virchows Arch. 2018 May;472(5):717-725. doi: 10.1007/s00428-017-2291-z. Epub 2018 Jan 15. Virchows Arch. 2018. PMID: 29333594
-
Hierarchical investigating the predictive value of p53, COX2, EGFR, nm23 in the post-operative patients with colorectal carcinoma.Oncotarget. 2017 Jan 3;8(1):954-966. doi: 10.18632/oncotarget.13512. Oncotarget. 2017. PMID: 27888614 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

