Optimization of ethylene glycol production from (D)-xylose via a synthetic pathway implemented in Escherichia coli
- PMID: 26336892
- PMCID: PMC4559361
- DOI: 10.1186/s12934-015-0312-7
Optimization of ethylene glycol production from (D)-xylose via a synthetic pathway implemented in Escherichia coli
Abstract
Background: Ethylene glycol (EG) is a bulk chemical that is mainly used as an anti-freezing agent and a raw material in the synthesis of plastics. Production of commercial EG currently exclusively relies on chemical synthesis using fossil resources. Biochemical production of ethylene glycol from renewable resources may be more sustainable.
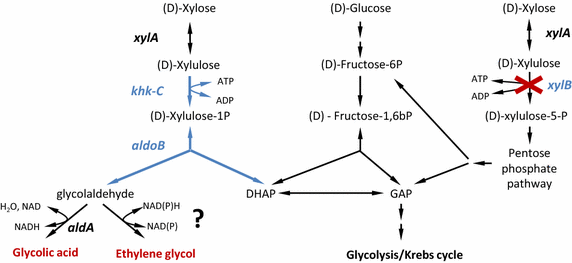
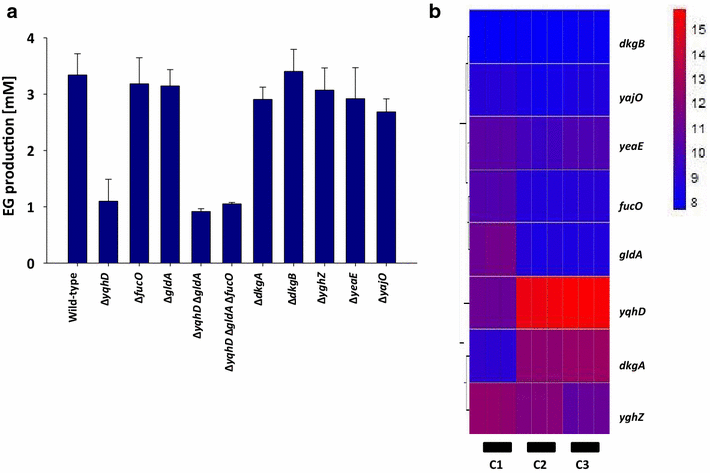
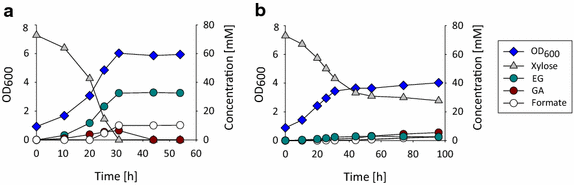
Results: Herein, a synthetic pathway is described that produces EG in Escherichia coli through the action of (D)-xylose isomerase, (D)-xylulose-1-kinase, (D)-xylulose-1-phosphate aldolase, and glycolaldehyde reductase. These reactions were successively catalyzed by the endogenous xylose isomerase (XylA), the heterologously expressed human hexokinase (Khk-C) and aldolase (Aldo-B), and an endogenous glycolaldehyde reductase activity, respectively, which we showed to be encoded by yqhD. The production strain was optimized by deleting the genes encoding for (D)-xylulose-5 kinase (xylB) and glycolaldehyde dehydrogenase (aldA), and by overexpressing the candidate glycolaldehyde reductases YqhD, GldA, and FucO. The strain overproducing FucO was the best EG producer reaching a molar yield of 0.94 in shake flasks, and accumulating 20 g/L EG with a molar yield and productivity of 0.91 and 0.37 g/(L.h), respectively, in a controlled bioreactor under aerobic conditions.
Conclusions: We have demonstrated the feasibility to produce EG from (D)-xylose via a synthetic pathway in E. coli at approximately 90 % of the theoretical yield.
Figures

References
-
- Bailey FE, Koleske JV. Poly(ethylene oxide) New York: Academic Press; 1976.
-
- Cox DP, Perlman D (1978) The biodegradation of polyethylene glycols. In: Advances in Applied Microbiology, vol 23. Academic Press, pp 173–194 - PubMed
-
- Mono-ethylene glycol. http://www.shell.com/global/products-services/solutions-for-businesses/c.... Accessed 15 May 2015
-
- Rebsdat S, Mayer D (2000) Ethylene glycol. In: Ullmann’s encyclopedia of industrial chemistry. Wiley-VCH Verlag GmbH & Co. KGaA
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

