NZ51, a ring-expanded nucleoside analog, inhibits motility and viability of breast cancer cells by targeting the RNA helicase DDX3
- PMID: 26337079
- PMCID: PMC4745771
- DOI: 10.18632/oncotarget.4898
NZ51, a ring-expanded nucleoside analog, inhibits motility and viability of breast cancer cells by targeting the RNA helicase DDX3
Abstract
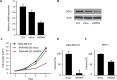
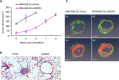
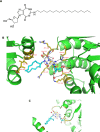
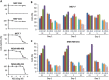
DDX3X (DDX3), a human RNA helicase, is over expressed in multiple breast cancer cell lines and its expression levels are directly correlated to cellular aggressiveness. NZ51, a ring-expanded nucleoside analogue (REN) has been reported to inhibit the ATP dependent helicase activity of DDX3. Molecular modeling of NZ51 binding to DDX3 indicated that the 5:7-fused imidazodiazepine ring of NZ51 was incorporated into the ATP binding pocket of DDX3. In this study, we investigated the anticancer properties of NZ51 in MCF-7 and MDA-MB-231 breast cancer cell lines. NZ51 treatment decreased cellular motility and cell viability of MCF-7 and MDA-MB-231 cells with IC50 values in the low micromolar range. Biological knockdown of DDX3 in MCF-7 and MDA-MB-231 cells resulted in decreased proliferation rates and reduced clonogenicity. In addition, NZ51 was effective in killing breast cancer cells under hypoxic conditions with the same potency as observed during normoxia. Mechanistic studies indicated that NZ51 did not cause DDX3 degradation, but greatly diminished its functionality. Moreover, in vivo experiments demonstrated that DDX3 knockdown by shRNA resulted in reduced tumor volume and metastasis without altering tumor vascular volume or permeability-surface area. In initial in vivo experiments, NZ51 treatment did not significantly reduce tumor volume. Further studies are needed to optimize drug formulation, dose and delivery. Continuing work will determine the in vitro-in vivo correlation of NZ51 activity and its utility in a clinical setting.
Keywords: RNA helicase DDX3; ring-expanded nucleoside.
Conflict of interest statement
The authors report no conflict of interest.
Figures

References
-
- NCI A Snapshot of Breast Cancer. Cancer Snapshots: Disease Focused and Other Snapshots. 2013
-
- Afifi M. Risk factors of breast cancer in women in Kelantan, Malaysia. Singapore Med J. 2006;47:551. author reply 552–553. - PubMed
-
- Salih AK, Fentiman IS. Breast cancer prevention: present and future. Cancer Treat Rev. 2001;27:261–273. - PubMed
-
- Lodovici M, Luceri C, Guglielmi F, Bacci CA, Victor F, Maria L, et al. Benzo(a)pyrene Diolepoxide (BPDE)-DNA Adduct Levels in Leukocytes of Smokers in Relation to Polymorphism of CYP1A1, GSTM1, GSTP1, GSTT1, and mEH. Cancer Epidemiol Biomarkers Prev. 2004;13:1342–1348. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

