Cholesterol Metabolism in CKD
- PMID: 26337134
- PMCID: PMC4658227
- DOI: 10.1053/j.ajkd.2015.06.028
Cholesterol Metabolism in CKD
Abstract
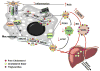
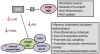
Patients with chronic kidney disease (CKD) have a substantial risk of developing coronary artery disease. Traditional cardiovascular disease (CVD) risk factors such as hypertension and hyperlipidemia do not adequately explain the high prevalence of CVD in CKD. Both CVD and CKD are inflammatory states and inflammation adversely affects lipid balance. Dyslipidemia in CKD is characterized by elevated triglyceride levels and high-density lipoprotein levels that are both decreased and dysfunctional. This dysfunctional high-density lipoprotein becomes proinflammatory and loses its atheroprotective ability to promote cholesterol efflux from cells, including lipid-overloaded macrophages in the arterial wall. Elevated triglyceride levels result primarily from defective clearance. The weak association between low-density lipoprotein cholesterol level and coronary risk in CKD has led to controversy over the usefulness of statin therapy. This review examines disrupted cholesterol transport in CKD, presenting both clinical and preclinical evidence of the effect of the uremic environment on vascular lipid accumulation. Preventative and treatment strategies are explored.
Keywords: Cholesterol transport; atherosclerosis; cardiovascular disease (CVD); chronic kidney disease (CKD); dyslipidemia; high-density lipoprotein (HDL); inflammation; lipid-lowering therapy; low-density lipoprotein (LDL); nontraditional risk factor; reactive oxygen species (ROS); statin therapy; uremic toxins.
Copyright © 2015 National Kidney Foundation, Inc. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension. 2003;42(5):1050–1065. - PubMed
-
- Hemmelgarn BR, Manns BJ, Lloyd A, et al. Alberta Kidney Disease Network. Relation between kidney function, proteinuria, and adverse outcomes. JAMA. 2010;303(5):423–429. - PubMed
-
- Manjunath G, Tighiouart H, Ibrahim H, MacLeod B, Salem DN, Griffith JL, Coresh J, Levey AS, Sarnak MJ. Level of kidney function as a risk factor for atherosclerotic cardiovascular outcomes in the community. J Am Coll Cardiol. 2003;41(1):47–55. - PubMed
-
- Go AS, Chertow GM, Fan D, McCullock CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

