Ca(2+) current facilitation determines short-term facilitation at inhibitory synapses between cerebellar Purkinje cells
- PMID: 26337248
- PMCID: PMC4650412
- DOI: 10.1113/JP270704
Ca(2+) current facilitation determines short-term facilitation at inhibitory synapses between cerebellar Purkinje cells
Abstract
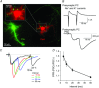
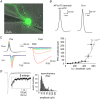
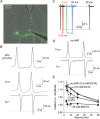
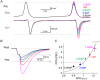
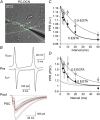
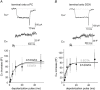
Key points: Short-term facilitation takes place at GABAergic synapses between cerebellar Purkinje cells (PCs). By directly patch clamp recording from a PC axon terminal, we studied the mechanism of short-term facilitation. We show that the Ca(2+) currents elicited by high-frequency action potentials were augmented in a [Ca(2+) ]i -dependent manner. The facilitation of synaptic transmission showed 4-5th power dependence on the Ca(2+) current facilitation, and was abolished when the Ca(2+) current amplitude was adjusted to be identical. Short-term facilitation of Ca(2+) currents predominantly mediates short-term facilitation at synapses between PCs.
Abstract: Short-term synaptic facilitation is critical for information processing of neuronal circuits. Several Ca(2+) -dependent positive regulations of transmitter release have been suggested as candidate mechanisms underlying facilitation. However, the small sizes of presynaptic terminals have hindered the biophysical study of short-term facilitation. In the present study, by directly recording from the axon terminal of a rat cerebellar Purkinje cell (PC) in culture, we demonstrate a crucial role of [Ca(2+) ]i -dependent facilitation of Ca(2+) currents in short-term facilitation at inhibitory PC-PC synapses. Voltage clamp recording was performed from a PC axon terminal visualized by enhanced green fluorescent protein, and the Ca(2+) currents elicited by the voltage command consisting of action potential waveforms were recorded. The amplitude of presynaptic Ca(2+) current was augmented upon high-frequency paired-pulse stimulation in a [Ca(2+) ]i -dependent manner, leading to paired-pulse facilitation of Ca(2+) currents. Paired recordings from a presynaptic PC axon terminal and a postsynaptic PC soma demonstrated that the paired-pulse facilitation of inhibitory synaptic transmission between PCs showed 4-5th power dependence on that of Ca(2+) currents, and was completely abolished when the Ca(2+) current amplitude was adjusted to be identical. Thus, short-term facilitation of Ca(2+) currents predominantly mediates short-term synaptic facilitation at synapses between PCs.
© 2015 The Authors. The Journal of Physiology © 2015 The Physiological Society.
Figures


Similar articles
-
The Mechanisms and Functions of Synaptic Facilitation.Neuron. 2017 May 3;94(3):447-464. doi: 10.1016/j.neuron.2017.02.047. Neuron. 2017. PMID: 28472650 Free PMC article. Review.
-
Axonal GABAA receptors depolarize presynaptic terminals and facilitate transmitter release in cerebellar Purkinje cells.J Physiol. 2017 Dec 15;595(24):7477-7493. doi: 10.1113/JP275369. Epub 2017 Nov 21. J Physiol. 2017. PMID: 29072780 Free PMC article.
-
beta-adrenergic receptor-mediated presynaptic facilitation of inhibitory GABAergic transmission at cerebellar interneuron-Purkinje cell synapses.J Neurophysiol. 2000 Oct;84(4):2016-25. doi: 10.1152/jn.2000.84.4.2016. J Neurophysiol. 2000. PMID: 11024094
-
Impact of the leaner P/Q-type Ca2+ channel mutation on excitatory synaptic transmission in cerebellar Purkinje cells.J Physiol. 2008 Sep 15;586(18):4501-15. doi: 10.1113/jphysiol.2008.156232. Epub 2008 Jul 31. J Physiol. 2008. PMID: 18669535 Free PMC article.
-
Pharmacology of the metabotropic glutamate receptor mediated current at the climbing fiber to Purkinje cell synapse.Prog Brain Res. 2005;148:299-306. doi: 10.1016/S0079-6123(04)48023-6. Prog Brain Res. 2005. PMID: 15661198 Review.
Cited by
-
Molecular Machines Regulating the Release Probability of Synaptic Vesicles at the Active Zone.Front Synaptic Neurosci. 2016 Mar 2;8:5. doi: 10.3389/fnsyn.2016.00005. eCollection 2016. Front Synaptic Neurosci. 2016. PMID: 26973506 Free PMC article. Review.
-
The Mechanisms and Functions of Synaptic Facilitation.Neuron. 2017 May 3;94(3):447-464. doi: 10.1016/j.neuron.2017.02.047. Neuron. 2017. PMID: 28472650 Free PMC article. Review.
-
Dynamic Factors for Transmitter Release at Small Presynaptic Boutons Revealed by Direct Patch-Clamp Recordings.Front Cell Neurosci. 2019 Jun 12;13:269. doi: 10.3389/fncel.2019.00269. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31249514 Free PMC article. Review.
-
Axonal GABAA receptors depolarize presynaptic terminals and facilitate transmitter release in cerebellar Purkinje cells.J Physiol. 2017 Dec 15;595(24):7477-7493. doi: 10.1113/JP275369. Epub 2017 Nov 21. J Physiol. 2017. PMID: 29072780 Free PMC article.
-
Molecular layer interneurons shape the spike activity of cerebellar Purkinje cells.Sci Rep. 2019 Feb 11;9(1):1742. doi: 10.1038/s41598-018-38264-1. Sci Rep. 2019. PMID: 30742002 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

