Endotoxin-induced inflammation down-regulates L-type amino acid transporter 1 (LAT1) expression at the blood-brain barrier of male rats and mice
- PMID: 26337286
- PMCID: PMC4559167
- DOI: 10.1186/s12987-015-0016-8
Endotoxin-induced inflammation down-regulates L-type amino acid transporter 1 (LAT1) expression at the blood-brain barrier of male rats and mice
Abstract
Background: We recently reported that bacterial lipopolysaccharide (LPS)-induced inflammation decreases the expression of the primary thyroid hormone transporters at the blood-brain barrier, organic anion-transporting polypeptide 1c1 (OATP1c1) and monocarboxylate transporter 8 (MCT8). L-type amino acid transporters 1 and 2 (LAT1 & LAT2) are regarded as secondary thyroid hormone transporters, and are expressed in cells of the blood-brain or blood-cerebrospinal fluid barrier and by neurons. The purpose of this study was to examine the effect of LPS-induced inflammation on the expression of LAT1 and LAT2, as these may compensate for the downregulation of OATP1c1 and MCT8.
Methods: LPS (2.5 mg/kg body weight) was injected intraperitoneally to adult, male, Sprague-Dawley rats and C57Bl/6 mice, which were euthanized 2, 4, 9, 24 or 48 h later. LAT1 and LAT2 mRNA expression were studied on forebrain sections using semiquantitative radioactive in situ hybridization. LAT1 protein levels in brain vessels were studied using LAT1 immunofluorescence. Statistical comparisons were made by the non-parametric Kruskal-Wallis and Dunn's tests.
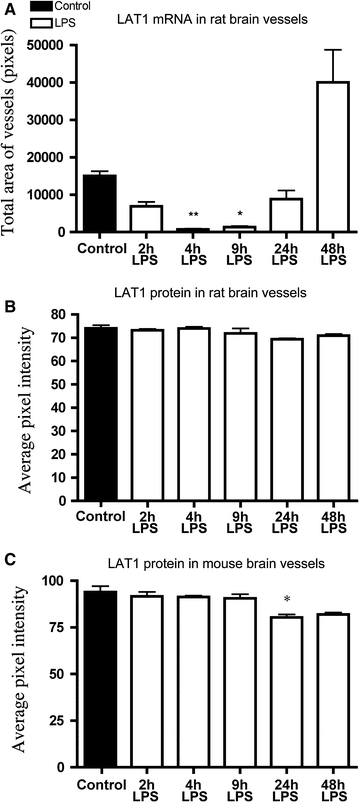
Results: In both species, LAT1 mRNA decreased in brain blood vessels as soon as 2 h after LPS injection and was virtually undetectable at 4 h and 9 h. During recovery from endotoxemia, 48 h after LPS injection, LAT1 mRNA in brain vessels increased above control levels. A modest but significant decrease in LAT1 protein levels was detected in the brain vessels of mice at 24 h following LPS injection. LPS did not affect LAT1 and LAT2 mRNA expression in neurons and choroid plexus epithelial cells.
Conclusions: The results demonstrate that LPS-induced inflammation rapidly decreases LAT1 mRNA expression at the blood-brain barrier in a very similar manner to primary thyroid hormone transporters, while changes in LAT1 protein level follow a slower kinetics. The data raise the possibility that inflammation may similarly down-regulate other blood-brain barrier transport systems at the transcriptional level. Future studies are required to examine this possibility and the potential pathophysiological consequences of inflammation-induced changes in blood-brain barrier transport functions.
Figures






Similar articles
-
Alzheimer's Disease Phenotype or Inflammatory Insult Does Not Alter Function of L-Type Amino Acid Transporter 1 in Mouse Blood-Brain Barrier and Primary Astrocytes.Pharm Res. 2018 Nov 28;36(1):17. doi: 10.1007/s11095-018-2546-7. Pharm Res. 2018. PMID: 30488131 Free PMC article.
-
Parallel regulation of thyroid hormone transporters OATP1c1 and MCT8 during and after endotoxemia at the blood-brain barrier of male rodents.Endocrinology. 2015 Apr;156(4):1552-64. doi: 10.1210/en.2014-1830. Epub 2015 Jan 16. Endocrinology. 2015. PMID: 25594699 Free PMC article.
-
Expression of the thyroid hormone transporters monocarboxylate transporter-8 (SLC16A2) and organic ion transporter-14 (SLCO1C1) at the blood-brain barrier.Endocrinology. 2008 Dec;149(12):6251-61. doi: 10.1210/en.2008-0378. Epub 2008 Aug 7. Endocrinology. 2008. PMID: 18687783
-
Pharmacokinetic role of L-type amino acid transporters LAT1 and LAT2.Eur J Pharm Sci. 2008 Oct 2;35(3):161-74. doi: 10.1016/j.ejps.2008.06.015. Epub 2008 Jul 5. Eur J Pharm Sci. 2008. PMID: 18656534 Review.
-
The pathophysiological consequences of thyroid hormone transporter deficiencies: Insights from mouse models.Biochim Biophys Acta. 2013 Jul;1830(7):3974-8. doi: 10.1016/j.bbagen.2012.04.009. Epub 2012 Apr 20. Biochim Biophys Acta. 2013. PMID: 22543196 Review.
Cited by
-
Variable proopiomelanocortin expression in tanycytes of the adult rat hypothalamus and pituitary stalk.J Comp Neurol. 2017 Feb 15;525(3):411-441. doi: 10.1002/cne.24090. Epub 2016 Sep 2. J Comp Neurol. 2017. PMID: 27503597 Free PMC article.
-
L-Type amino acid transporter 1 as a target for drug delivery.Pharm Res. 2020 May 6;37(5):88. doi: 10.1007/s11095-020-02826-8. Pharm Res. 2020. PMID: 32377929 Free PMC article. Review.
-
Effect of Lipopolysaccharide and TNFα on Neuronal Ascorbic Acid Uptake.Mediators Inflamm. 2021 Jul 3;2021:4157132. doi: 10.1155/2021/4157132. eCollection 2021. Mediators Inflamm. 2021. PMID: 34285658 Free PMC article.
-
Alzheimer's Disease Phenotype or Inflammatory Insult Does Not Alter Function of L-Type Amino Acid Transporter 1 in Mouse Blood-Brain Barrier and Primary Astrocytes.Pharm Res. 2018 Nov 28;36(1):17. doi: 10.1007/s11095-018-2546-7. Pharm Res. 2018. PMID: 30488131 Free PMC article.
-
Impaired T3 uptake and action in MCT8-deficient cerebral organoids underlie Allan-Herndon-Dudley syndrome.JCI Insight. 2024 Feb 20;9(7):e174645. doi: 10.1172/jci.insight.174645. JCI Insight. 2024. PMID: 38376950 Free PMC article.
References
-
- Ceballos A, Belinchon MM, Sanchez-Mendoza E, Grijota-Martinez C, Dumitrescu AM, Refetoff S, et al. Importance of monocarboxylate transporter 8 for the blood-brain barrier-dependent availability of 3,5,3′-triiodo-l-thyronine. Endocrinology. 2009;150(5):2491–2496. doi: 10.1210/en.2008-1616. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

