Spatio-temporal relief from hypoxia and production of reactive oxygen species during bud burst in grapevine (Vitis vinifera)
- PMID: 26337519
- PMCID: PMC4578006
- DOI: 10.1093/aob/mcv123
Spatio-temporal relief from hypoxia and production of reactive oxygen species during bud burst in grapevine (Vitis vinifera)
Abstract
Background and aims: Plants regulate cellular oxygen partial pressures (pO2), together with reduction/oxidation (redox) state in order to manage rapid developmental transitions such as bud burst after a period of quiescence. However, our understanding of pO2 regulation in complex meristematic organs such as buds is incomplete and, in particular, lacks spatial resolution.
Methods: The gradients in pO2 from the outer scales to the primary meristem complex were measured in grapevine (Vitis vinifera) buds, together with respiratory CO2 production rates and the accumulation of superoxide and hydrogen peroxide, from ecodormancy through the first 72 h preceding bud burst, triggered by the transition from low to ambient temperatures.
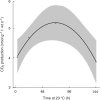
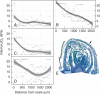
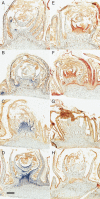
Key results: Steep internal pO2 gradients were measured in dormant buds with values as low as 2·5 kPa found in the core of the bud prior to bud burst. Respiratory CO2 production rates increased soon after the transition from low to ambient temperatures and the bud tissues gradually became oxygenated in a patterned process. Within 3 h of the transition to ambient temperatures, superoxide accumulation was observed in the cambial meristem, co-localizing with lignified cellulose associated with pro-vascular tissues. Thereafter, superoxide accumulated in other areas subtending the apical meristem complex, in the absence of significant hydrogen peroxide accumulation, except in the cambial meristem. By 72 h, the internal pO2 gradient showed a biphasic profile, where the minimum pO2 was external to the core of the bud complex.
Conclusions: Spatial and temporal control of the tissue oxygen environment occurs within quiescent buds, and the transition from quiescence to bud burst is accompanied by a regulated relaxation of the hypoxic state and accumulation of reactive oxygen species within the developing cambium and vascular tissues of the heterotrophic grapevine buds.
Keywords: Bud burst; ROS; Vitis vinifera; development; ecodormancy; grapevine; hypoxia; meristem; oxygen partial pressure; quiescence; reactive oxygen species; respiration; superoxide.
© The Author 2015. Published by Oxford University Press on behalf of the Annals of Botany Company.
Figures


Similar articles
-
Developmental control of hypoxia during bud burst in grapevine.Plant Cell Environ. 2018 May;41(5):1154-1170. doi: 10.1111/pce.13141. Epub 2018 Feb 28. Plant Cell Environ. 2018. PMID: 29336037
-
Hydrogen cyanamide breaks grapevine bud dormancy in the summer through transient activation of gene expression and accumulation of reactive oxygen and nitrogen species.BMC Plant Biol. 2016 Sep 15;16(1):202. doi: 10.1186/s12870-016-0889-y. BMC Plant Biol. 2016. PMID: 27627883 Free PMC article.
-
The initiation of bud burst in grapevine features dynamic regulation of the apoplastic pore size.J Exp Bot. 2020 Jan 7;71(2):719-729. doi: 10.1093/jxb/erz200. J Exp Bot. 2020. PMID: 31037309 Free PMC article.
-
Redox regulation of meristem quiescence: outside/in.J Exp Bot. 2024 Oct 16;75(19):6037-6046. doi: 10.1093/jxb/erae161. J Exp Bot. 2024. PMID: 38676562 Free PMC article. Review.
-
On the language and physiology of dormancy and quiescence in plants.J Exp Bot. 2016 May;67(11):3189-203. doi: 10.1093/jxb/erw138. Epub 2016 Apr 6. J Exp Bot. 2016. PMID: 27053719 Review.
Cited by
-
Reactive Oxygen Species and Antioxidants in Postharvest Vegetables and Fruits.Int J Food Sci. 2020 Dec 10;2020:8817778. doi: 10.1155/2020/8817778. eCollection 2020. Int J Food Sci. 2020. PMID: 33381540 Free PMC article. Review.
-
Abscisic acid (ABA) and low temperatures synergistically increase the expression of CBF/DREB1 transcription factors and cold-hardiness in grapevine dormant buds.Ann Bot. 2019 Mar 14;123(4):681-689. doi: 10.1093/aob/mcy201. Ann Bot. 2019. PMID: 30418484 Free PMC article.
-
Roles for Light, Energy, and Oxygen in the Fate of Quiescent Axillary Buds.Plant Physiol. 2018 Feb;176(2):1171-1181. doi: 10.1104/pp.17.01479. Epub 2017 Dec 4. Plant Physiol. 2018. PMID: 29203560 Free PMC article. Review.
-
Lipid Body Dynamics in Shoot Meristems: Production, Enlargement, and Putative Organellar Interactions and Plasmodesmal Targeting.Front Plant Sci. 2021 Jul 21;12:674031. doi: 10.3389/fpls.2021.674031. eCollection 2021. Front Plant Sci. 2021. PMID: 34367200 Free PMC article.
-
Complexity of Abiotic Stress Stimuli: Mimicking Hypoxic Conditions Experimentally on the Basis of Naturally Occurring Environments.Methods Mol Biol. 2023;2642:23-48. doi: 10.1007/978-1-0716-3044-0_2. Methods Mol Biol. 2023. PMID: 36944871
References
-
- Antcliff AJ, May P. 1961. Dormancy and bud burst in sultana vines. Vitis 3: 1–14.
-
- Benitez-Alfonso Y, Jackson D, Maule A. 2011. Redox regulation of intercellular transport. Protoplasma 248: 131–140. - PubMed
-
- Borisjuk L, Rolletschek H. 2009. The oxygen status of the developing seed. New Phytologist 182: 17–30. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

