Organic-inorganic hybrid nanoflowers: types, characteristics, and future prospects
- PMID: 26337651
- PMCID: PMC4559159
- DOI: 10.1186/s12951-015-0118-0
Organic-inorganic hybrid nanoflowers: types, characteristics, and future prospects
Abstract
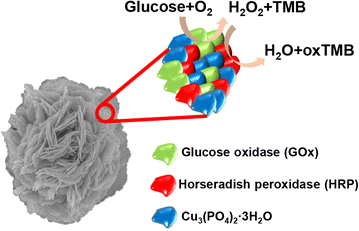
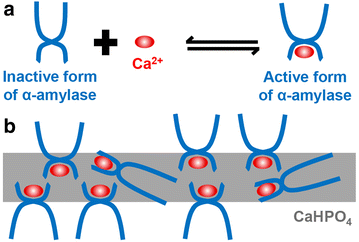
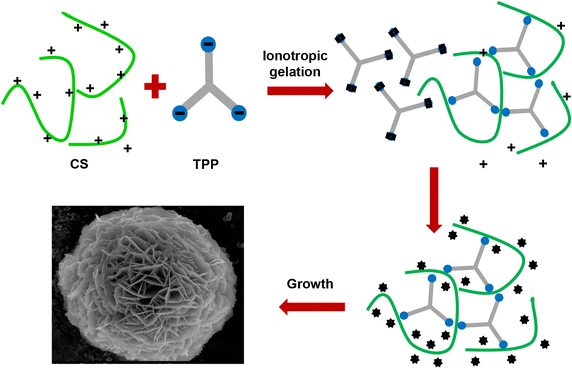
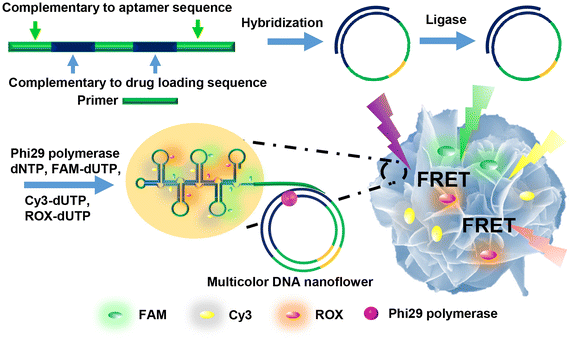
Organic-inorganic hybrid nanoflowers, a newly developed class of flower-like hybrid nanoparticles, have received much attention due to their simple synthesis, high efficiency, and enzyme stabilizing ability. This article covers, in detail, the types, structural features, mechanism of formation, and bio-related applications of hybrid nanoflowers. The five major types of hybrid nanoflowers are discussed, i.e., copper-protein, calcium-protein, and manganese-protein hybrid nanoflowers, copper-DNA hybrid nanoflowers, and capsular hybrid nanoflowers. The structural features of these nanoflowers, such as size, shape, and protein ratio generally determine their applications. Thus, the specific characteristics of hybrid nanoflowers are summarized in this review. The interfacial mechanism of nanoflower formation is examined in three steps: first, combination of metal ion and organic matter; second, formation of petals; third, growth of nanoflowers. The explanations provided herein can be utilized in the development of innovative approaches for the synthesis of hybrid nanoflowers for prospective development of a plethora of hybrid nanoflowers. The future prospects of hybrid nanoflowers in the biotechnology industry, medicine, sensing, and catalysis are also discussed.
Figures




References
-
- Lattuada M, Hatton TA. Synthesis, properties and applications of Janus nanoparticles. Nano Today. 2011;6(3):286–308. doi: 10.1016/j.nantod.2011.04.008. - DOI
-
- Kim J, Grate JW. Single-enzyme nanoparticles armored by a nanometer-scale organic/inorganic network. Nano Lett. 2003;3(9):1219–1222. doi: 10.1021/nl034404b. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

