Hypoxia-induced carbonic anhydrase IX facilitates lactate flux in human breast cancer cells by non-catalytic function
- PMID: 26337752
- PMCID: PMC4559800
- DOI: 10.1038/srep13605
Hypoxia-induced carbonic anhydrase IX facilitates lactate flux in human breast cancer cells by non-catalytic function
Abstract
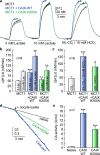
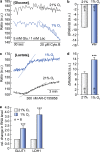
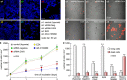
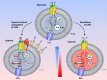
The most aggressive tumour cells, which often reside in hypoxic environments, rely on glycolysis for energy production. Thereby they release vast amounts of lactate and protons via monocarboxylate transporters (MCTs), which exacerbates extracellular acidification and supports the formation of a hostile environment. We have studied the mechanisms of regulated lactate transport in MCF-7 human breast cancer cells. Under hypoxia, expression of MCT1 and MCT4 remained unchanged, while expression of carbonic anhydrase IX (CAIX) was greatly enhanced. Our results show that CAIX augments MCT1 transport activity by a non-catalytic interaction. Mutation studies in Xenopus oocytes indicate that CAIX, via its intramolecular H(+)-shuttle His200, functions as a "proton-collecting/distributing antenna" to facilitate rapid lactate flux via MCT1. Knockdown of CAIX significantly reduced proliferation of cancer cells, suggesting that rapid efflux of lactate and H(+), as enhanced by CAIX, contributes to cancer cell survival under hypoxic conditions.
Figures



References
-
- Hanahan D. & Weinberg R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011). - PubMed
-
- Schulze A. & Harris A. L. How cancer metabolism is tuned for proliferation and vulnerable to disruption. Nature 491, 364–373 (2012). - PubMed
-
- Parks S. K., Chiche J. & Pouysségur J. Disrupting proton dynamics and energy metabolism for cancer therapy. Nat. Rev. Cancer 13, 611–623 (2013). - PubMed
-
- Brahimi-Horn M. C., Bellot G. & Pouysségur J. Hypoxia and energetic tumour metabolism. Curr. Opin. Genet. Dev . 21, 67–72 (2011). - PubMed
-
- Gatenby R. A. & Gillies R. J. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer 4, 891–899 (2004). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

