Therapeutic drug monitoring of eculizumab: Rationale for an individualized dosing schedule
- PMID: 26337866
- PMCID: PMC4966484
- DOI: 10.1080/19420862.2015.1086049
Therapeutic drug monitoring of eculizumab: Rationale for an individualized dosing schedule
Abstract
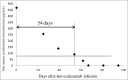
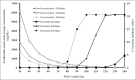
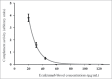
The annual cost of eculizumab maintenance therapy in paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic-uremic syndrome (aHUS) exceeds $300,000 per patient. A better understanding of eculizumab pharmacokinetics and subsequent individual dose adjustment could reduce this cost. We measured the trough eculizumab concentration in 9 patients with maintenance therapy (aHUS, n = 7; PNH, n = 2) and determined: 1) the intra- and inter-individual variability; 2) the influence of weight on eculizumab pharmacokinetics; and 3) the rate of elimination of eculizumab following discontinuation. A one-compartment model was developed to describe the pharmacokinetics of eculizumab and predicted complement activity by body weight. Trough eculizumab concentrations were >50 µg/mL in 9/9, >100 µg/mL in 8/9, and >300 µg/mL in 5/9 of patients. Intra-individual variability was low but eculizumab concentrations, closely correlated with patient weight (R(2) = 0.66, p = 0.034), varied broadly (55 ± 12 to 733 ± 164 µg/mL). Pharmacokinetic modeling showed that the elimination half-life varied greatly, with an increase from 7.8 d in a patient weighing 100 kg to 19.5 d in a 40 kg patient. We predicted that infusions of 1200 mg could be spaced every 4 or 6 weeks in patients weighing <90 and <70 kg, respectively. In this pilot study, the current recommended use of a fixed eculizumab dose for maintenance therapy is associated with excessively high trough concentrations in many patients. Further prospective larger studies are now required to support an individualized schedule adjusted for patient weight and based on the observed trough serum eculizumab concentration.
Keywords: Eculizumab; Hemolytic uremic syndrome; Paroxysmal nocturnal hemoglobinuria; Pharmacokinetics; Thrombotic microangiopathy.
Figures


References
-
- Thomas TC, Rollins SA, Rother RP, Giannoni MA, Hartman SL, Elliott EA, Nye SH, Matis LA, Squinto SP, Evans MJ. Inhibition of complement activity by humanized anti-C5 antibody and single-chain Fv. Mol Immunol 1996; 33:1389-401; PMID:9171898; http://dx.doi.org/10.1016/S0161-5890(96)00078-8 - DOI - PubMed
-
- Rother RP, Rollins SA, Mojcik CF, Brodsky RA, Bell L. Discovery and development of the complement inhibitor eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria Nat Biotechnol 2007; 25:1256-64; PMID:17989688; http://dx.doi.org/10.1038/nbt1344 - DOI - PubMed
-
- Burwick RM, Burwick NR, Feinberg BB. Eculizumab fails to inhibit generation of C5a in vivo. Blood 2014; 124:3502-3; PMID:25431478; http://dx.doi.org/10.1182/blood-2014-07-589366 - DOI - PubMed
-
- Hillmen P, Young NS, Schubert J, Brodsky RA, Socié G, Muus P, Röth A, Szer J, Elebute MO, Nakamura R, et al.. The Complement Inhibitor Eculizumab in Paroxysmal Nocturnal Hemoglobinuria. N Engl J Med 2006; 355:1233-43; PMID:16990386; http://dx.doi.org/10.1056/NEJMoa061648 - DOI - PubMed
-
- Legendre CM, Licht C, Muus P, Greenbaum LA, Babu S, Bedrosian C, Bingham C, Cohen DJ, Delmas Y, Douglas K,. Terminal Complement Inhibitor Eculizumab in Atypical Hemolytic–Uremic Syndrome. N Engl J Med 2013; 368:2169-81; PMID:23738544; http://dx.doi.org/10.1056/NEJMoa1208981 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
